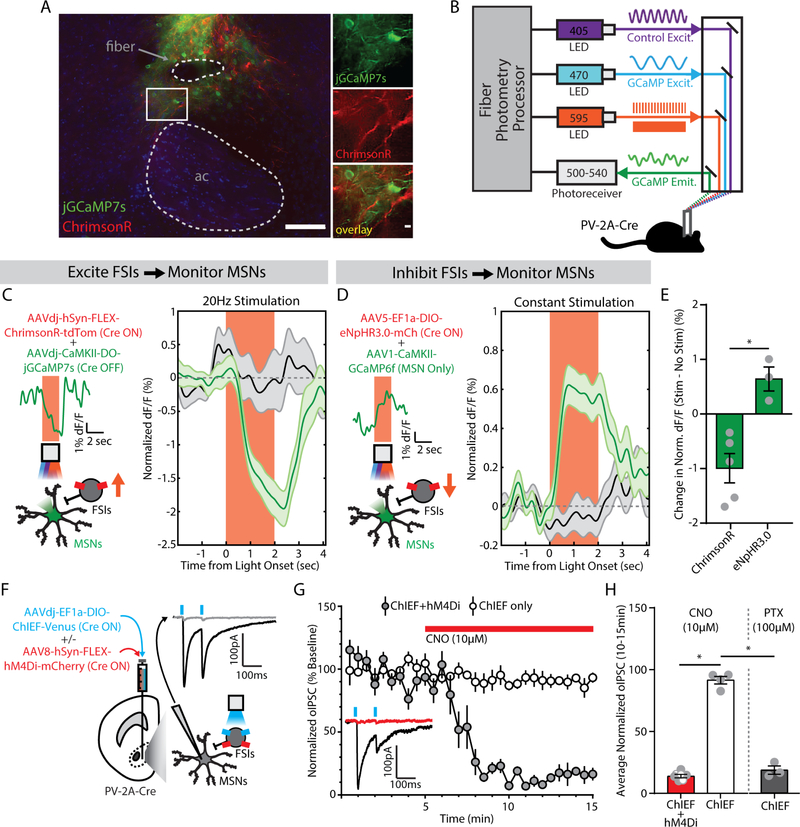
Figure 4. Fast-spiking interneurons (FSIs) inhibit medium spiny neurons (MSNs) in the nucleus accumbens (NAc) core.
(A) (left) Visualization of fiber photometry implant placed above FSIs expressing ChrimsonR (red) and MSNs expressing jGCaMP7s (green; ac, anterior commissure; scale bar, 100 μm). (right) Magnified image of FSI-MSN juxtaposition (scale bar, 10 μm). (B) Setup for simultaneous red-shifted optogenetic manipulation (pulsed for ChrimsonR, constant for eNpHR3.0) and fiber photometry monitoring in vivo. (C) (left) Viral co-injection of Cre-dependent ChrimsonR to excite FSIs with Cre-inactivated jGCaMP7s to monitor MSNs. Insets show photometry signal on a single representative trial. (right) MSN signal decreases from baseline during FSI excitation (green, n=20 trials/mouse), compared to no stimulation trials (black, n=20 trials/mouse) (n=5 male mice). (D) (left) Viral co-injection of Cre-dependent NpHR3.0 to inhibit FSIs with CaMKII-promoted GCaMP6f to monitor MSNs. Insets show photometry signal on a single representative trial. (right) MSN signal increases from baseline during FSI inhibition (green, n=20 trials/mouse), compared to no stimulation trials (black, n=20 trials/mouse) (n=3 male mice). (E) Significant difference in MSN signal between FSI excitation and inhibition. *p<0.05, main effect of group. (F) Viral co-injection of Cre-dependent ChIEF, with or without Cre-dependent hM4Di. Inset shows an optogenetically-evoked inhibitory postsynaptic current (oIPSC, black) blocked by picrotoxin (100 μM, gray). (G) oIPSCs in slices co-expressing hM4Di were reduced by bath application of clozapine-N-oxide (CNO, 10μM, red bar) (ChIEF only, n=4 male cells; ChIEF+hM4Di, n=7 cells; 3 female, 4 male). Inset shows an example trace before (black) and after (red) CNO, from a slice expressing ChIEF+hM4Di. (H) oIPSCs were significantly reduced by chemogenetic inhibition (red) or picrotoxin (PTX, gray; n=3 male cells). *p<0.05, main effect of group.