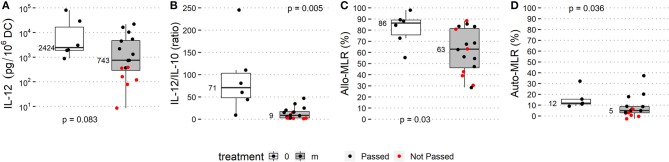
Figure 3.
Treatment prior to monocyte harvest and immunostimulatory properties of manufactured DCs. Manufacturing subgroup from monocytes harvested after MTD-based therapy potentially interfering with monocyte biology (listed in Supplementary Table 1; “m” treatment, gray box plots) and manufacturing subgroup from monocytes from untreated patients or after non-interfering treatment (“0” treatment, white box plots) were compared based on QC parameters: (A) IL-12 production, (B) IL-12/IL-10 production ratio, (C) allo-MLR and (D) auto-MLR. Median values are shown for each parameter for each treatment subgroup. Black dots show QC results of manufactured DCs that passed quality control, and red dots show results of manufactured DCs that did not pass quality control.