Abstract
Background
Hepatitis D virus is a small defective RNA virus that requires the presence of hepatitis B virus infection to infect a person. Hepatitis D is a difficult‐to‐treat infection. Several clinical trials have been published on the efficacy of interferon alpha for hepatitis D virus (HDV) infection. However, there are few randomised trials evaluating the effects of interferon alpha, and it is difficult to judge any benefit of this intervention from the individual trials.
Objectives
To evaluate the beneficial and harmful effects of interferon alpha for patients with chronic hepatitis D.
Search methods
We identified relevant for the review randomised clinical trials by electronic searches in the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until May 2011. We also checked the bibliographic references of identified randomised trials, textbooks, and review articles in order to find randomised trials not identified by the electronic searches.
Selection criteria
Randomised clinical trials evaluating interferon alpha versus placebo or no intervention for patients with chronic hepatitis D infection.
Data collection and analysis
Two authors assessed the trials and extracted data on mortality, virologic, biochemical, and histological response as well as adverse events at end of treatment and six months or more after completing treatment. The analyses were performed using the intention‐to‐treat principle including all randomised participants irrespective of follow‐up. Drop‐outs, withdrawals, and non‐compliance were considered as treatment failures. Data were analysed with fixed‐ and random‐effects models. Reported results were based on fixed‐effect model except in cases where statistical significance varied between the two models.
Main results
Six randomised trials fulfilled the inclusion criteria. Two hundred and one randomised participants (male = 174) were included. The risk of bias in all the included trials was high. Five trials compared interferon alpha with no treatment in the control group. One of these trials had two treatment arms with a higher dose and lower dose of interferon alpha and a no‐treatment control group. We analysed both treatment regimens as a single group in a primary analysis and as separate groups in the subgroup analysis of different interferon dosages. The sixth trial compared only a higher dose of interferon alpha with a lower dose.
Meta‐analysis of five trials comparing interferon alpha with no‐treatment control group included 169 participants. There were seven drop‐outs in the treatment group and nine in the control group. One patient out of 92 (1.1%) died in the interferon alpha group compared with zero out of 77 (0.0%) in the no‐intervention control group (risk ratio (RR)) 3.00; 95% confidence interval (CI) 0.14 to 66.5). Interferon alpha led to failure of end of treatment virological response in 62/92 (67.4%) of the patients compared with 71/77 (92.2%) in the untreated controls (RR 0.76, 95% CI 0.66 to 0.87, P = 0.0001 by fixed‐effect model and RR 0.71, 95% CI 0.43 to 1.16, P = 0.17 by random‐effects model). Failure of normalisation of alanine aminotransferase (ALT) at the end of treatment was seen in 60/92 (65.2%) patients treated with interferon alpha versus 76/77 (98.7%) in the control group (RR 0.69, 95% CI 0.59 to 0.80, P < 0.00001). Sustained virological response was not achieved in 76/92 (82.6%) of patients on interferon compared with 73/77 (94.8%) of controls (RR 0.89, 95% CI 0.80 to 0.98, P = 0.02). Serum alanine aminotransferase was abnormal in 81/92 (88.0%) treated with interferon alpha patients at six months post‐treatment follow‐up compared with 76/77 (98.7%) in controls (RR 0.92, 95% CI 0.84 to 0.99, P = 0.04). There was no significant histological improvement in 67/92 (72.8%) patients treated with interferon alpha compared with 65/77 (84.4%) in controls (RR 0.86, 95% CI 0.74 to 1.00, P = 0.06).
Two trials comparing a higher dose of interferon alpha with the lower dose showed no significant difference in sustained virological response (76.7% compared with 90.0%) (RR 0.85, 95% CI 0.68 to 1.07, P = 0.16). Adverse events such as flu‐like symptoms, asthenia, weight loss, alopecia, thrombocytopenia, and leukopenia were reported in all these trials and the adverse events were related to interferon alpha. These were common and sometimes severe. One patient in the treatment group was reported to have died by suicide towards the end of the study period.
Authors' conclusions
Interferon alpha does not seem to cure hepatitis D in most patients. The agent seems effective in suppressing viral and liver disease activity in some patients, but this improvement is not sustained in the majority of patients. We cannot exclude overestimation of benefits and underestimation of harms due to high risk of bias (systematic errors) and high risk play of chance (random errors). Therefore, more randomised trials with large sample sizes and less risk of bias are needed before interferon can be recommended or refuted.
Keywords: Female; Humans; Male; Antiviral Agents; Antiviral Agents/adverse effects; Antiviral Agents/therapeutic use; Hepatitis D, Chronic; Hepatitis D, Chronic/drug therapy; Interferon‐alpha; Interferon‐alpha/adverse effects; Interferon‐alpha/therapeutic use; Randomized Controlled Trials as Topic
Interferon alpha for patients with chronic hepatitis D
Hepatitis D virus is unique in that it can only infect a person who is already infected by hepatitis B virus. Chronic hepatitis D is a difficult‐to‐treat infection. Several antiviral and immunomodulating agents have been evaluated in treatment of hepatitis D. However, with the exception of interferon, all of them proved ineffective. This meta analysis of six randomised clinical trials of interferon shows that even Interferon alpha is not an ideal drug for this infection. Among the 169 participants included in primary meta analysis, interferon alpha induced loss of virus, normalisation of liver tests, and improvement in the liver biopsy in more patients compared with those who were left untreated. Unfortunately, most of these patients did not have sustained response after stopping treatment. Additional analysis of two trials comparing a higher dose of interferon alpha with lower dose among randomly assigned participants showed no significant difference in outcome between the two groups. There were differences in dosage and duration of interferon alpha used among included trials as well as some other methodological weakness which places a high risk of bias in this meta analysis.
Background
Hepatitis D virus (or delta virus) (HDV) is a defective small single‐stranded circular RNA virus that requires the helper function of hepatitis B virus (HBV) for viral assembly and propagation (Rizzetto 1977). Acute infection with HDV acquired by coinfection with HBV is often severe. However, most patients achieve usually a complete recovery and only 2% of the patients progress to chronicity (Farci 2003). Superinfection of HDV in persons with HBV infection leads to progressive disease and cirrhosis in approximately 80% of patients (Rizzetto 2003). Cirrhosis develops earlier in HDV‐infected patients than patients infected only with chronic HBV (Rizzetto 1983; Hughes 2011). Up to 5% of the world's population is infected with HBV, and probably 5% of the HBV carriers have HDV superinfection (Gaeta 2001). Accordingly, about 15 million people may have chronic hepatitis D infection.
HDV is difficult to eradicate as most of the possible therapeutic targets are normal cellular proteins. The sole enzymatic activity that HDV possesses is a ribozyme that autocleaves the circular RNA, producing a linear molecule (Sharmeen 1988). Concomitant infection with an RNA (HDV) and a DNA (HBV) virus makes chronic hepatitis D more difficult to treat than chronic hepatitis B alone. As with hepatitis B, poor results were obtained in the treatment of hepatitis D with immunosuppressive and immunostimulant drugs (Arrigoni 1983; Rizzetto 1983). The mechanism of action of interferon in chronic hepatitis D is poorly understood. In HDV transfected hepatoma cell lines, HDV replication was not affected by interferon (Ottobrelli 1991; Ilan 1992). In vitro experiments apparently contrast with the results observed in patients, in whom response to interferon is often characterised by a concomitant reduction in HDV viraemia and in alanine aminotransferase (ALT) levels, suggesting a direct antiviral effect of interferon on HDV.
Several clinical trials on the long‐term administration of interferon were undertaken in the late 1980s (Farci 1994; Malaguarnera 1996). The response, assessed by the normalisation of serum alanine aminotransferase (ALT) levels and clearance of serum HDV RNA varied widely. Moreover, it occurred at different times from the beginning of treatment, sometimes even after discontinuation of interferon. The proportion of patients with response and relapse seemed proportional to the dose of interferon (Di Bisceglie 1990; Madejon 1994). Sustained responses were unusual and often incomplete, showing persistently normal ALT despite the recrudescence of HDV viraemia. Concomitant sustained biochemical and virological responses were usually accompanied by the clearance of serum hepatis B surface antigen (HBsAg) and seroconversion to anti‐HBs antibody (Battegay 1994).
Farci et al demonstrated that interferon alpha, given in a dose of 9 million units three times a week for 48 weeks, was generally well tolerated and resulted in clearance of serum HDV RNA, normal ALT values, and histologic improvement in 50% of patients with chronic hepatitis D (Farci 1994). A complete biochemical response persisted for up to four years in half the patients who had normal ALT values at the end of therapy, whereas the effects on viral replication were not sustained in these patients. Niro et al reviewed the trials on the treatment of hepatitis D and concluded that the medical treatment of chronic hepatitis D rested on interferon, which should be administered at high doses (9 to 10 million units three times a week) to patients with compensated liver disease and as soon as chronic HDV disease was diagnosed (Niro 2005). Treatment should be prolonged for 12 months as response, ie, clearance of HDV RNA and normalisation of ALT levels, can be delayed and sometimes occur after the end of the treatment.
There is one previous meta‐analysis available dealing with interferon alpha for hepatitis D (Hadziyannis 1991). It is based on reduction in ALT levels as the primary outcome measure. We could not find any comprehensive meta‐analyses that evaluated interferon alpha for chronic hepatitis D in terms of mortality and virological, biochemical, and histological responses at the end of treatment and the end of the follow‐up. Therefore, we felt the need to perform the present Cochrane systematic review.
Objectives
To evaluate the beneficial and harmful effects of interferon alpha in the treatment of patients with chronic hepatitis D infection.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials irrespective of language, publication date, or blinding.
Types of participants
Inclusion criteria
Included in this analysis were patients with chronic hepatitis D infection. That is, patients with detectable serum HDV RNA for at least six months with inactive or active HBV carrier state, and active inflammatory disease, ie, persistent or intermittent raised activities of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) above the upper limit of normal values (Farci 1994). Patients with compensated delta virus related cirrhosis were included as well. Patients with hepatitis C virus and HIV co‐infection, alcoholism, patients using immunosuppressive drugs, and liver transplanted patients were also considered for inclusion in subgroup analyses.
Exclusion criteria
Patients with acute hepatitis D (ie, acute co‐infection with HDV and HBV or acute HDV superinfection on HVB).
Types of interventions
Standard interferon alpha or pegylated interferon alpha versus placebo or no intervention. Interferon alpha could be administered in any dosage via the subcutaneous or intramuscular route. We considered for inclusion also trials with co‐interventions if these were administered equally to the relevant intervention groups.
Types of outcome measures
Primary outcomes
1. Mortality. 2. Failure of sustained virologic response: number of patients with positive HDV RNA at six months or more after treatment. 3. Adverse events: any unfavourable or unintended sign (including an abnormal laboratory finding), symptom, or disease (new or exacerbated) associated with use of a medicinal product (ICH‐GCP 1997). These include serious or minor; expected or unexpected; and study‐related, possibly study‐related, or not study‐related events.This also included patients developing decompensation during interferon therapy. 4. Quality of life.
Secondary outcomes 1. Failure of sustained biochemical response: failure of normalisation of ALT and/or AST levels at six months or more after treatment. 2. Failure of histological response: failure of improvement of inflammatory activity as assessed by liver biopsy.
Search methods for identification of studies
Electronic searches
Relevant randomised clinical trials were identified by electronic searches in the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003). Last date of search was May 31, 2011. Search strategies applied to the individual electronic databases with the time span of the searches are given in Appendix 1.
Searching other resources
We also checked the bibliographic references of identified randomised trials, textbooks, and review articles in order to find randomised trials not identified by the electronic searches.
Data collection and analysis
Selection of studies
We retrieved the full paper articles for assessment, and review authors applied the inclusion criteria to the trials of interest to the review independently of each other. There were no disagreements among the authors.
Data extraction and management
Two authors (ZAB and MAK) extracted the following prespecified characteristics of all included randomised clinical trials independently. In case of discrepancy, the opinion of the third reviewer (WJA or MSA) was sought in order to reach consensus. Data included:
Participants: age, sex, ethnic origin, form(s) of transmission, previous antiviral treatment, presence of cirrhosis at entry, criteria used to classify chronic hepatitis D, number of patients randomised, reasons for withdrawal from the trial.
Interventions: dosage and duration of therapy, and method of administration, intervention in the control group, and any co‐interventions.
Outcomes: as listed above under outcome measures.
Assessment of risk of bias in included studies
The methodological quality of a trial can affect the estimate of intervention efficacy (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Gluud 2011). Risk of bias of the randomised clinical trials was assessed using the definitions of following domains (Higgins 2011).
Sequence generation ‐ Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are also adequate if performed by an independent adjudicator. ‐ Uncertain risk of bias: the trial is described as randomised but the method of sequence generation was not specified. ‐ High risk of bias: the sequence generation method is not, or may not be, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients are inadequate and were excluded for the assessment of benefits but not for the assessment of harms.
Allocation concealment ‐ Low risk of bias: allocation was controlled by a central and independent randomisation unit, serially numbered, opaque and sealed envelopes, or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment. ‐ Uncertain risk of bias: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. ‐ High risk of bias: if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. Quasi‐randomised studies were excluded for the assessment of benefits but not for the assessment of harms.
Blinding ‐ Low risk of bias: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. ‐ Uncertain risk of bias: the trial was described as blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. ‐ High risk of bias: the trial was not blinded, so that the allocation was known during the trial.
Incomplete outcome data ‐ Low risk of bias: the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals. ‐ Uncertain risk of bias: the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. ‐ High risk of bias: the number or reasons for dropouts and withdrawals were not described.
Selective outcome reporting ‐ Low risk of bias: pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. ‐ Uncertain risk of bias: not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not. ‐ High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
If a trial obtained the judgement 'low risk of bias' in all the six bias risk domains, then it was categorised as a trial with low risk of bias for the purpose of data analyses. In all other cases, the trial was categorised as a trial with high risk of bias.
Dealing with missing data
An intention‐to‐treat analysis included all randomised participants. Any missing observations were assumed to have a poor outcome.
Assessment of heterogeneity
Review Manager software (RevMan) was used for the data analysis (Deeks 2011; RevMan 2011). Continuous outcomes are expressed as mean differences (MD) with 95% confidence intervals (CI). For dichotomous variables we calculated relative risk with 95% CI. Intention‐to‐treat principle was applied everywhere.
Heterogeneity between trials was explored by considering the bias risk of trials, clinical setting, and patients involved. Chi‐squared test for heterogeneity was used to provide an indication of between trials heterogeneity. In addition, the degree of heterogeneity observed in the results was quantified using the I‐squared statistic (Higgins 2003). The heterogeneity statistic I2, interpreted as "the percentage of variability due to heterogeneity between studies rather than sampling error depends on precision, that is, the size of the studies included" (Rücker 2008).
Assessment of reporting biases
Regression asymmetry test to assess funnel plot asymmetry was to be employed to indicate the presence of bias (Egger 1997). However, we did not identify a sufficient number of trials in order to draw it.
Data synthesis
We analysed the data with both fixed‐effect (DeMets 1987) and random‐effects (DerSimonian 1986) model meta‐analyses. In case there was no difference in statistical significance between the results obtained with the two models, we presented the results of the fixed‐effect model analyses. Otherwise, we presented the results of both analyses. The I2 statistic was presented as a measure of the percentage of variation due to heterogeneity rather than chance (Higgins 2003). The analyses were performed using the intention‐to‐treat principle including all randomised participants irrespective of follow‐up. Drop‐outs, withdrawals and non‐compliance were considered as treatment failures. Details are given in 'early stopping' section.
From the data generated by each included randomised clinical trial, risk ratio was calculated for categorical outcome data and standardised mean differences for continuous data along with their 95% CI. The results from comparable groups of trials were pooled into statistical meta‐analysis using RevMan (RevMan 2011). Heterogeneity between combined trials was tested using standard chi‐square test. Where statistical pooling was not possible, the findings were summarised by listing and narrating.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses: ‐ lower median dose of interferon compared to upper median dose of interferon (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4).
Analysis 3.1.
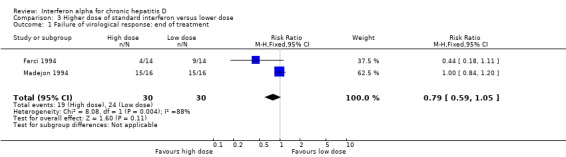
Comparison 3 Higher dose of standard interferon versus lower dose, Outcome 1 Failure of virological response: end of treatment.
Analysis 3.2.
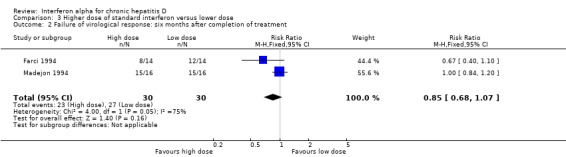
Comparison 3 Higher dose of standard interferon versus lower dose, Outcome 2 Failure of virological response: six months after completion of treatment.
Analysis 3.3.
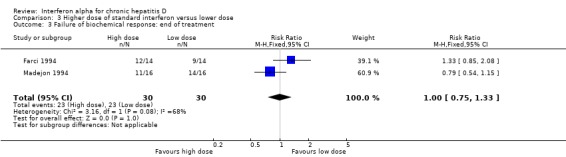
Comparison 3 Higher dose of standard interferon versus lower dose, Outcome 3 Failure of biochemical response: end of treatment.
Analysis 3.4.
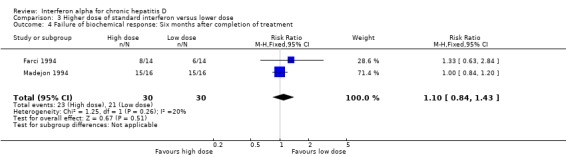
Comparison 3 Higher dose of standard interferon versus lower dose, Outcome 4 Failure of biochemical response: Six months after completion of treatment.
We could not perform the below listed subgroup analyses either because there were no sufficient data in the included trials or because the topic was not addressed.
‐ trials with low risk of bias compared to trials with high risk of bias; ‐ trials with short follow‐up (less than six months) compared to trials with long follow‐up (more than twelve months); ‐ pretreatment levels of ALT and/or AST; ‐ types of interferon administered; ‐ adult compared to paediatric patients; ‐ patients with coinfection with hepatitis C virus or HIV compared to patients without coinfection; ‐ patients with alcohol problems compared to patients without coinfection; ‐ patients with immunosuppressive drugs compared to patients without coinfection; ‐ patients with liver transplantation compared to patients without liver transplantation.
Results
Description of studies
Results of the search
The search strategy identified 35 studies for consideration, out of which six trials fulfilled the criteria for inclusion and were used for our meta‐analyses (Figure 1).
Figure 1.
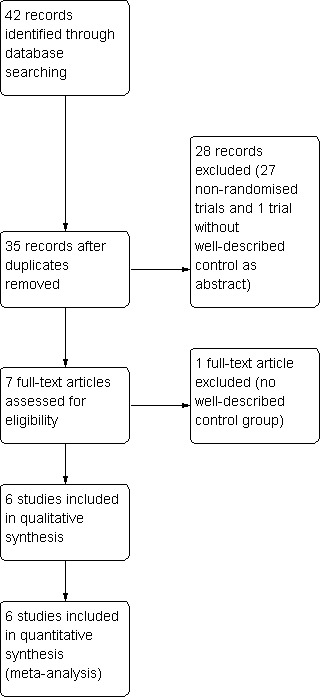
Study flow diagram.
Included studies
Six trials with 201 randomised participants provided data for analysis; 174 were males and 27 were women (Table 4). Five trials compared interferon monotherapy versus no intervention control (Porres 1989; Rosina 1989; Rosina 1991;Farci 1994; Gaudin 1995). A total of 169 patients were included in these five trials; 92 in the intervention group and 77 in the no intervention group. The baseline characteristics of the patient sample included in the trials did not show any substantial differences between the groups in the individual trials as well as across the trials. These trials had methodological heterogeneity in terms of dosage regimen of interferon alpha and duration of administration of interferon (refer to Characteristics of included studies table). The duration of treatment was one year in three trials, six months in one (Porres 1989), and three months in another trial (Rosina 1989). Out of the five trials mentioned above, four trials (Porres 1989;Rosina 1989; Rosina 1991;Gaudin 1995) randomised patients to interferon alpha versus no intervention in the control group. The fifth trial (Farci 1994) had three groups; two interferon alpha intervention groups and a control group with no treatment. The interferon groups tested a lower dose (3 million units thrice a week) and a higher dose (9 million units thrice a week). We analysed both treatment regimens as a single group in a primary analysis (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5) and as separate groups in the analysis of different interferon dosages. The remaining trial (Madejon 1994) compared a higher dose of interferon alpha (18 million units thrice a week for 6 months, 9 million units thrice a week for 1 month, 6 million units tiw for 1 month, 3 million units thrice a week for 4 months) versus a lower dose (3 million units daily for 3 months then 1.5 million units daily for 9 months) of interferon alpha. We have included the Madejon 1994 trial in a subgroup analysis (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4),
Table 1.
Male:female ratio
| Study ID | Male | Female |
| Porres 1989 | 15 | 5 |
| Rosina 1989 | 22 | 2 |
| Rosina 1991 | 54 | 7 |
| Farci 1994 | 35 | 7 |
| Madejon 1994 | 26 | 6 |
| Gaudin 1995 | 22 | 0 |
| Total | 174 | 27 |
Analysis 2.1.
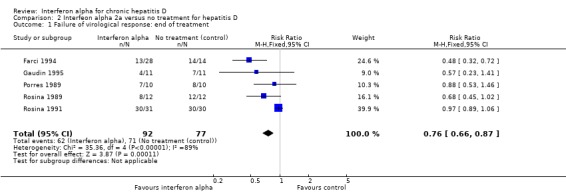
Comparison 2 Interfeon alpha 2a versus no treatment for hepatitis D, Outcome 1 Failure of virological response: end of treatment.
Analysis 2.2.
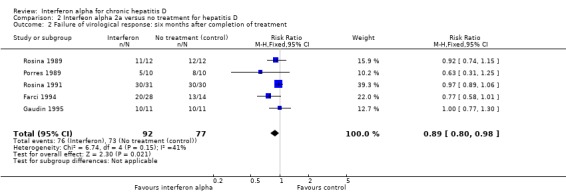
Comparison 2 Interfeon alpha 2a versus no treatment for hepatitis D, Outcome 2 Failure of virological response: six months after completion of treatment.
Analysis 2.3.
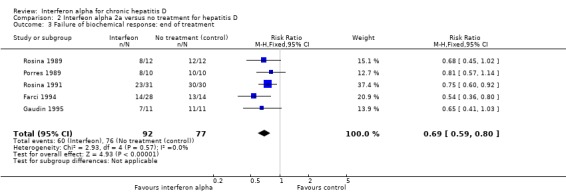
Comparison 2 Interfeon alpha 2a versus no treatment for hepatitis D, Outcome 3 Failure of biochemical response: end of treatment.
Analysis 2.4.
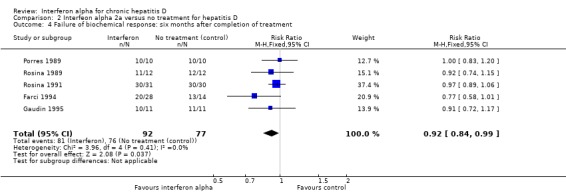
Comparison 2 Interfeon alpha 2a versus no treatment for hepatitis D, Outcome 4 Failure of biochemical response: six months after completion of treatment.
Analysis 2.5.
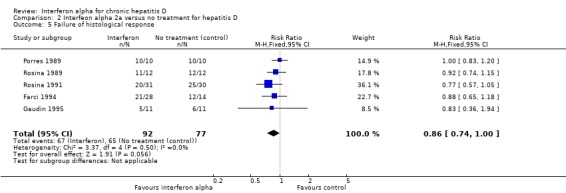
Comparison 2 Interfeon alpha 2a versus no treatment for hepatitis D, Outcome 5 Failure of histological response.
Excluded studies
Among the 29 excluded studies, two studies lacked a well‐described control group (Borghesio 1995;Di Marco 1996). The remainder were not randomised clinical trials or addressed different topics (Characteristics of excluded studies).
Risk of bias in included studies
The risk of bias domains were utilised to evaluate the individual trial for risk of systematic error (Higgins 2011). The results are summarised in Figure 2 and Figure 3. All the trials had high risk of bias.
Figure 2.
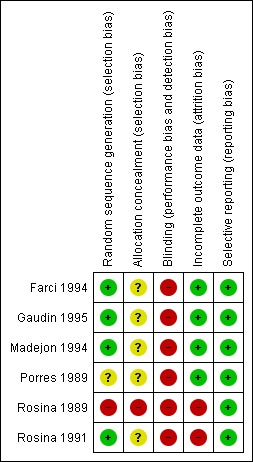
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Figure 3.
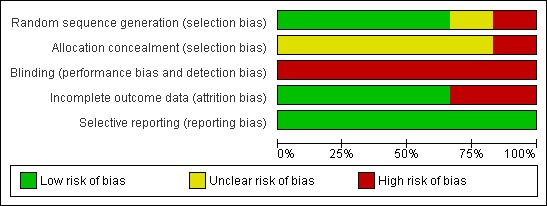
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
Allocation
All of the included trials randomly allocated patients to comparison groups. Four trials had computer‐generated allocation sequence, and the process was regarded as adequate. In Porres 1989 and Rosina 1989, the authors did not give sufficient details regarding the method used and simply stated that the patients were randomly allocated into two groups.
Regarding concealment of allocation, Farci 1994 was the only positive exception using computer‐generated sealed envelopes. Even this trial does not mention whether the envelopes were opaque or not, or if they were consecutively numbered. None of the other included trials mentioned a specific process of concealment. This was regarded as lack of concealment.
Blinding
None of the included trials utilised blinding.
Incomplete outcome data
The included trials generally accounted for all the participants. Outcome variables were identical, and outcomes data were complete in nearly all the trials. The only exception to this was Porres 1989, where the outcome variables were not clearly defined. Nonetheless, we attempted to assess the results based on established criteria for end of treatment response and sustained virological response. These criteria provided a fair representation of the trials. Wherever missing data were found, assessment was based on an intention‐to‐treat principle, and a failure of treatment was presumed. This happened mostly in case of a second biopsy, which was not done in all the participants.
Selective reporting
All the included trials were considered free of selective reporting. Every trial reported on the predetermined outcomes for each patient included, according to the trial report. We acknowledge, however, that we did not have access to any of the trial protocols.
Other potential sources of bias
Baseline imbalance The baseline characteristics of patients between experimental and control groups were similar. The only possible exception was the apparent disparity in the duration of disease between the two groups in Madejon 1994 (62.6 ± 10.4 months in low dose interferon versus 49.7 ± 9.8 in high dose group).
Early stopping In Rosina 1991 interferon was discontinued permanently in five patients. Reasons were ulcer at the injection site in one patient, acute icteric hepatitis in another, and non‐compliance in three. Eight untreated patients were withdrawn from the control group for noncompliance. In Farci 1994, one patient was lost to follow‐up in the control group. Madejon 1994: Drop outs and withdrawals were seven; three from the low dose and four from the high dose group. Reasons were return to active drug abuse (n = 2), neuropsychiatric disorder (n = 2), thrombocytopoaenia (n = 2), and voluntary withdrawal (n = 1).Gaudin 1995: Therapy was discontinued in two patients; one at four months because of induction of hyperthyroidsm and the other at 11 months because of committed suicide. There were no drop‐outs or early stopping in Porres 1989 and Rosina 1989.
Unit‐of‐analysis bias There was a clear methodological heterogeneity among the trials as already alluded to earlier. In one trial (Farci 1994), multiple treatment groups were employed. The groups were then redefined to ensure simplified pair‐wise comparison for a representative analysis. This may have resulted in a potential unit‐of‐analysis bias for the meta‐analysis.
Effects of interventions
Interferon alpha versus no intervention
Mortality
One patient in the interferon alpha group was reported to have died by suicide towards the end of the study period (Gaudin 1995). No other trials reported any deaths during the treatment or follow‐up period (Analysis 1.1).
Analysis 1.1.
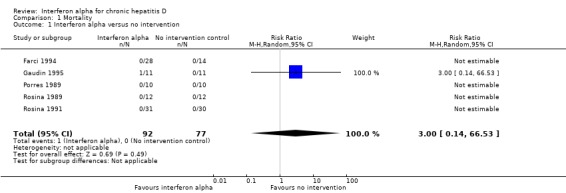
Comparison 1 Mortality, Outcome 1 Interferon alpha versus no intervention.
Failure of sustained virologic response
A total of 169 patients were included in five trials; 92 in the intervention group and 77 in the no intervention group. There were seven drop‐outs in the treatment group and nine in the control group. By intention to treat analysis, failure of sustained virological response (SVR) at six months follow‐up was observed in 82.6% in patients on interferon alpha compared with 94.8% in controls (RR 0.89, 95% CI 0.80 to 0.98, P = 0.02) (Analysis 2.2). I2 of 41% also indicated a more homogenous distribution among the meta‐analysed groups.
Interferon alpha administration led to failure of end of treatment response in 67.4% of the patients compared with 92.2% in controls based on clearance of HDV DNA (RR 0.76, 95% CI 0.66 to 0.87, P = 0.0001 by fixed‐effect model, RR 0.71, 95% CI 0.43 to 1.16, not significant by random‐effects model) (Analysis 2.1). There was considerable heterogeneity in pooled results (I2 = 89%, P < 0.00001).
Adverse events
All the trials included in the analysis reported on adverse events related to administration of interferon alpha. We classified adverse events into two groups, viz, (a) adverse events not requiring any modification in interferon therapy (Table 5), and (b) adverse events requiring modification or termination of treatment (Table 6). Among the first set of complications, nearly every patient experienced flu‐like symptoms across the trials. Other commonly reported adverse events included anorexia, nausea, weight loss, alopaecia, leukopaenia, and thrombocytopaenia.
Table 2.
Adverse events related to interferon therapy
| Study ID | Adverse events listed | Percentage of patients |
| Porres 1989 | Flu‐like symptoms Weight loss Leukopoenia Thrombocytopoenia | 100 40 30 40 |
| Rosina 1989 | Flu‐like symptoms Transient hair loss Herpes labialis Granuloopoenia | 100 33 25 67 |
| Rosina 1991 | Flu‐like symptoms Fatigue Weight loss Alopaecia Nausea/Anorexia Vomiting Impaired consciousness Rhinorrhea | 100 100 100 17 35 6 3 3 |
| Farci 1994 | Flu‐like symptoms Asthenia Alopaecia Anemia | 100 50 43 4 |
| Madejon 1994 | Asthenia Anorexia Fever Weight loss Arthralgias Hair loss Headache Itching | 56 50 47 47 41 38 38 12 |
| Gaudin 1995 | Flu‐like symptoms Leukopoenia Thrombocytopoenia Hyperthyroidism Death (by suicide) | 100 100 100 10 10 |
Table 3.
2 Adverse events requiring dose modification or termination of interferon therapy
| Study ID | Events* | Total number of participants | % |
| Porres 1989 | 0 | 10 | 0 |
| Rosina 1989 | 0 | 12 | 0 |
| Rosina 1991 | 16 | 31 | 51.6 |
| Farci 1994 | 2 | 28 | 7.1 |
| Gaudin 1995 | 4 | 11 | 36.4 |
| Madejon 1994 | 7 | 32 | 21.9 |
| Total | 29 | 124 | 23.4 |
Total number of participants are the patients who received interferon. Events represent number of participants experiencing adverse events requiring dose modification or termination of therapy
Quality of life
None of the trials reported on the quality of life.
Failure of sustained biochemical response
At six months follow‐up, ALT was abnormal in 88.0% treated patients versus 98.7% controls (RR 0.92, 95% CI 0.84 to 0.99, P = 0.04) (Analysis 2.4). There was no significant heterogeneity among the trials on these counts (end of treatment: I2 = 0%, P = 0.57, sustained biochemical response I2 = 0%, P = 0.41). Failure of normalisation of ALT at the end of treatment was seen in 65.2% patients treated with interferon alpha versus 98.7% in the no intervention control group (RR 0.69, 95% CI 0.59 to 0.80, P < 0.00001) (Analysis 2.3).
Failure of histological response
Assessment of histological response was restricted on a number of accounts. Different trials were unable to repeat biopsies on all the participants; this was especially true of untreated control group participants. An assumption of no improvement in histology was thus presumed in those with missing biopsy. Additionally, reporting of histological findings and grading of severity were performed on different scales among the trials. We decided to assess histological outcome as a dichotomous variable with improvement noticed or no improvement noticed among participants of a trial. There was no histological improvement in 72.8% patients treated with interferon alpha compared with 84.4% in controls (RR 0.86, 95% CI 0.74 to 1.00, P = 0.06) (Analysis 2.5). There was no heterogeneity among the trials (I2 0 %, P = 0.50).
Subgroup analysis
We searched the trials for data on patients with hepatitis C virus and HIV co‐infection, alcoholism, patients using immunosuppressive drugs, and liver transplanted patients in order to perform subgroup analyses. However, none of the trials fulfilling the inclusion criteria of the review protocol provided specific details of the individual patients in this regard, and these subgroup analyses could not be performed. However, we performed the subgroup analysis comparing high median dose of interferon alpha with low dose. Data about improvement in the quality of life were also not available.
Interferon alpha high dose compared with interferon alpha low dose
Two trials, comparing a higher cumulative dose regimen with a lower dose regimen of interferon alpha, failed to achieve sustained virologic response in 76.7% of the patients with higher dose compared to 90% with the lower dose (RR 0.85, 95% CI 0.68 to 1.07), but this difference was not statistically significant (P = 0.16) (Analysis 3.1; Analysis 3.2) (Farci 1994; Madejon 1994). There was also no significant difference in biochemical response between high dose and low dose interferon groups (Analysis 3.3; Analysis 3.4).
Discussion
Summary of main results
Chronic hepatitis D aggravates the natural course of hepatitis B infection. It is difficult to treat hepatitis D. Interferon is the only treatment for chronic hepatitis D. Randomised clinical trials based on interferon therapy were conducted in late 1980s and early 1990s. These trials are few. The response evaluated was clearance of HDV RNA (virological response), normalisation of ALT (biochemical response), and histological improvement based on liver biopsy. We found absence of significant sustained virological response, improvement in ALT, and histological improvement in our analysis. We observed, however, a potential effect of interferon on end of treatment virological and biochemical responses. These observations are hampered by the risk of significant errors (bias) and the risk of random error (play of chance). We, therefore, tend to agree with Hughes et al (Hughes 2011): interferon alpha, standard or pegylated, seems to be the only effective therapy available so far for HDV, though it may not be an ideal treatment. This therapy may not cure the infection in all patients, but it may potentially benefit in some patients. Such potential effects come at a price: increased risk of adverse events and of costs.
Interesting, although sustained virological response is not achieved in all patients, biochemical response appears to correlate with improvement in liver histology. The beneficial effect lasts even beyond the termination of therapy. A 2 to 14 year follow‐up study of patients from Farci 1994 showed that high doses of interferon alpha‐2a (9 million units thrice a week) significantly improved the long‐term clinical outcome and survival of patients with chronic hepatitis D, even though the majority had active cirrhosis before the onset of therapy (Farci 2004). These patients had a sustained decrease in HDV replication, leading to clearance of HDV RNA and, eventually, hepatitis B virus (HBV) in some patients, as well as a dramatic improvement in liver histology with respect to activity grade and fibrosis stage. So, the clearance of serum HDV RNA associated with loss of HBsAg may occur years after discontinuation of treatment (Lau 1999). In patients, who do not clear HDV RNA but do show biochemical and histological response, interferon probably induces less pathogenic mutants (Ottobrelli 1991).
With the development of pegylated interferon, uncontrolled studies on hepatitis D have appeared in literature. Castelnau et al showed an end of treatment virological response of 57% (8/14) with pegylated interferon alpha 2b 1.5 microgram per kg and sustained virological response of 43% (Castelnau 2006). However, another study of 12 patients using the same dose showed a sustained virological response of only 17% (Erhardt 2006). Negative polymerase chain reaction (PCR) or decrease of more than three logs in HDV RNA level at six months of intervention is correlated with sustained virological response. In an international trial done by Wedemeyer and colleagues (HIDIT‐1), pegylated interferon alpha 2a had a significant antiviral efficacy against HDV, with 28% achieving a sustained virological response (Wedemeyer 2011).
HBsAg is required for production of viral hepatitis D particles, and active suppression of this antigen seems, therefore, a must. Few trials have compared the effectiveness of combination of one of the nucleoside analogues or ribavirin with standard or pegylated interferon versus interferon alone. These combinations failed to show advantage of using combination over interferon monotherapy (Niro 2006). Available therapies do not effectively suppress the surface antigen but do have some effect in reducing its level. For example, lamivudine and famciclovir individually are ineffective against HDV (Yurdaydin 2002; Niro 2005a). Four randomised trials comparing interferon monotherapy with lamivudine, adefovir dipivoxil, or ribavirin combination with interferon failed to show improvement in the virological and biochemical responses over interferon monotherapy (Gunsar 2005; Canbakan 2006; Yurdaydin 2008; Wedemeyer 2011). Inclusion of these more recent trials in the analysis is beyond the scope of what was defined in the objectives of the current review.
There is a need to develop new therapies effective directly against HDV. There are few reports of clearance of HBsAg in up to 11% of the patients of hepatitis B with one year of pegylated interferon therapy. Thus, monitoring of HBsAg levels along with HDV RNA levels would be recommended in future trials to evaluate response. Ideally treatment should be continued until the loss of HBsAg. Monitoring HDV RNA levels could help in predicting the response and adjusting the duration of therapy just as done for hepatitis C (Lok 2007; EASL 2009; Ghany 2009) The HIDIT‐1 trial showed that combination of pegylated interferon with adefovir dipivoxil was superior to interferon monotherapy in reducing HBsAg levels (Wedemeyer 2011). There is a need for randomised trials comparing pegylated interferon monotherapy with its combination with more powerful nucleos(t)ide analogues and for longer duration. In the future, drugs directly acting on HDV life cycle such as antisense oligonucleotides (Chen 1997), prenylation inhibitors (Bordier 2003), and HBV/HDV virus entry inhibitors (Petersen 2008) would also have some role alone, or probably in combination with pegylated interferon.
Overall completeness and applicability of evidence
From the available trials' data we may conclude that interferon alpha potentially seems effective in suppressing viral activity and decreasing liver disease activity in some patients, but the inhibitory effect is temporary, and improvement is not sustained in the majority of patients. From the limited data available, it is not possible to find out any predictive factors or determinants of response. Due to low sustained response, it seems difficult to accept this agent as standard of care for hepatitis D. The possibility that pegylated interferon might be more effective needs further evaluation in clinical trials. The reason is that all included trials had high risk of bias (systematic errors) and high risk of play of chance (random errors) and we cannot exclude outcome measure reporting bias as well as publication bias.
Quality of the evidence
Data from the available randomised trials were difficult to compare due to the small number of trials and differences in the dose, duration, and agents used in combination. In addition to methodological heterogeneity, only one subgroup analysis could be conducted. All trials were unblinded, and several of them also showed other bias risks. Allocation concealment was not observed or was not clear. However, these trials were considered free from selective reporting and incomplete outcome data were adequately addressed by most of the trials. Assessment was based on an intention‐to‐treat principle. Possibility of publication bias cannot be excluded, and the risk of bias in the included trials was high.
Potential biases in the review process
We did not limit our search to English language publications only. However, the databases we searched contain less number of journals indexed from the developing world. We tried to retrieve the unpublished data by going through the abstracts from liver meetings. Data of an unpublished trial were generously provided by their authors. However, during the process of writing the review, the study got published (Wedemeyer 2011). This meta‐analysis is based on a small number of trials, with each trial comprising a small sample size compounded by the differing dosage and duration of interferon administration. These limitations carry over into our analysis, and in our opinion, restricts definitive conclusion regarding this treatment.
Agreements and disagreements with other studies or reviews
Other reviews have also highlighted the limited efficacy of interferon alpha against hepatitis D (Malaguarnera 1996; Farci 2003; Niro 2005; Farci 2007; Wedemeyer 2010). They have mentioned the individual studies and have drawn conclusions without performing meta‐analysis. There is one meta‐analysis available which has been done by Hadziyannis (Hadziyannis 1991). However, it is based on biochemical response, and used reduction in ALT levels as the primary outcome measure. While we did the systematic review comparing the trials in clinically relevant outcome measures such as mortality, virological, biochemical, and histological responses at the end of treatment and the end of the follow‐up period and calculated the meta‐analysis results. We also did subgroup analysis comparing high dose with low dose of interferon.
Authors' conclusions
Administration of interferon to patients with chronic hepatitis D can neither be supported nor refuted. Interferon may benefit some patients through long‐term remission. The patients run the risk of adverse events and serious adverse events. Newer therapies are needed.
Randomised clinical trials are needed to compare interferon alpha versus placebo (or no placebo), pegylated interferon with standard interferon, to determine duration of therapy, ie, 12 months versus 18 or 24 months, to document any improvement in response with combination of newer, more powerful nucleoside or nucleotide analogues, and to evaluate combination of pegylated interferon with prenylation inhibitors or HBV/HDV virus entry inhibitors.
Other possible interventions ought to be assessed in chronic hepatitis D. A possible candidate could be a HBV/HDV virus entry inhibitor. In the future, trials ought to be reported according to the recommendations of CONSORT (http://www.consort‐statement.org/).
Acknowledgements
We express our gratitude to Christian Gluud for motivating us to write the protocol for this review and reviewing the manuscript, Dimitrinka Nikolova for her valuable comments and assistance in writing, editing and reviewing, and Sarah Louise Klingenberg for performing the electronic searches.
Peer Reviewers: Steven A Gonzalez, USA; Jalal Poorolajal, Iran; Luit Penninga, Denmark. Contact Editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time Span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | May 2011 | (*interferon* OR peg‐ifn OR pegasus OR pegasys OR pegintron OR 'viraferon peg') AND 'hepatitis D' |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 2, 2011 | #1 MeSH descriptor Interferon‐alphaexplode all trees in MeSH products #2 interferon* or pegylated interferon or peginterferon or peg‐ifn or pegas*s or pegintron or viraferon peg in All Fieldsin all products #3 (#1 OR #2) #4 MeSH descriptor Hepatitis Dexplode all trees in MeSH products #5 hepatitis NEXT d in All Fieldsin all products #6 (#4 OR #5) #7 (#3 AND #6) |
| MEDLINE (Ovid SP) | 1950 to May 2011 | 1. exp Interferon‐alpha/ 2. (interferon* or pegylated interferon or peginterferon or peg‐ifn or pegas*s or pegintron or viraferon peg).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 3. 1 or 2 4. exp Hepatitis D/ 5. hepatitis d.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 6. 4 or 5 7. 6 and 3 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 9. 8 and 7 |
| EMBASE (Ovid SP) | 1980 to May 2011 | 1. exp Alpha Interferon/ 2. (interferon* or pegylated interferon or peginterferon or peg‐ifn or pegas*s or pegintron or viraferon peg).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 3. 1 or 2 4. exp Delta Agent Hepatitis/ 5. hepatitis d.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. 4 or 5 7. 6 and 3 8. (random* or placebo* or blind* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. 8 and 7 |
| Science Citation Index Expanded (http://apps.isiknowledge.com) | 1900 to May 2011 | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analysis) #3 #2 AND #1 #2 TS=(hepatitis D) #1 TS=(interferon* or pegylated interferon or peginterferon or peg‐ifn or pegas*s or pegintron or viraferon peg) |
Data and analyses
Comparison 1.
Mortality
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interferon alpha versus no intervention | 5 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.14, 66.53] |
Comparison 2.
Interfeon alpha 2a versus no treatment for hepatitis D
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of virological response: end of treatment | 5 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.87] |
| 2 Failure of virological response: six months after completion of treatment | 5 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 0.98] |
| 3 Failure of biochemical response: end of treatment | 5 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.80] |
| 4 Failure of biochemical response: six months after completion of treatment | 5 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.84, 0.99] |
| 5 Failure of histological response | 5 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 1.00] |
Comparison 3.
Higher dose of standard interferon versus lower dose
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of virological response: end of treatment | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 2 Failure of virological response: six months after completion of treatment | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.07] |
| 3 Failure of biochemical response: end of treatment | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.75, 1.33] |
| 4 Failure of biochemical response: Six months after completion of treatment | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.43] |
Differences between protocol and review
Not all the subgroup analyses could be performed due to non‐availability of data of individual patients.
An extreme case‐analyses was not conducted and dropouts were considered as failures in both groups.
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Randomised clinical trial. Sample size: no justification. Intention‐to‐treat: yes. Interim analyses: no. |
|
| Participants | Patients with chronic hepatitis D from Spain (n = 20). Inclusion criteria: positive HDV‐IgM antibody, biopsy proven chronic hepatitis with intrahepatic delta antigen. Exclusion criteria: previous antiviral or steroid therapy, signs of active HBV infection, ie, HBeAg or HBV DNA. Treatment and comparison groups similar at the start of study. |
|
| Interventions | Control: no treatment (n =10). Experimental 1: interferon alfa‐2c 10 million units/square meter twice a week (n =10). Duration: six months. Follow‐up: 9 months post treatment. | |
| Outcomes | Loss of anti‐HDV IgM. Loss of HDV RNA. Normalisation of ALT. Improvement in liver histology. |
|
| Notes | Three patients with positive anti‐HIV (two in control and one in treatment group. Subgroup analyses were not performed in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "The patients were randomly allocated into two groups." Method not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded trial design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | " All the patients remained on the treatment until the end of the treatment period". |
| Selective reporting (reporting bias) | Low risk | Outcome measures adequately reported. |
| Methods | Randomised clinical trial. Sample size: no justification. Intention‐to‐treat: yes. |
|
| Participants | Patients with chronic hepatitis D (n =24) from Italy. Inclusion criteria: positive HDV antibody, elevated ALT for one year, histological evidence of chronic hepatitis and positive HDV antigen in liver Bx done within two months Exclusion criteria: Not mentioned. |
|
| Interventions | Control: no treatment (n =12). Experimental 1: interferon alfa‐2b 5 million units (MU) t.i.w. (n =12). Duration: 3 months. Follow‐up: 9 months post treatment. Liver biopsy at enrolment and end of post treatment follow‐up. |
|
| Outcomes | Normalisation of ALT. Improvement in liver histology. Decrease in HDV RNA level. Loss of HDV RNA. Loss of HDV antigen in serum. |
|
| Notes | Second liver biopsy was done in only 4/12 controls. Two patients in the control group were negative for HDV RNA at the time of enrolment, end of treatment, and end of follow‐up and do not fulfil the criteria for response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote" the patients were matched for age and sex, randomly assigned to a treated or control group". It is mentioned in the abstract but not described in the "Materials and Methods" section. In the comparison table, standard deviations for the age at baseline were not mentioned, and baseline ALT levels were not given. |
| Allocation concealment (selection bias) | High risk | No. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded trial design. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Second liver biopsy was done in only 4/12 controls. |
| Selective reporting (reporting bias) | Low risk | Outcome measures adequately reported. |
| Methods | Sample size: not calculated. Intention‐to‐treat: yes. |
|
| Participants | Patients with chronic hepatitis D (n =61) from Italy. Inclusion criteria: positive HDV antibody, elevated ALT for one year, histological evidence of chronic hepatitis or cirrhosis, and positive staining for HDAg on liver Bx done within six months . Exclusion criteria were: previous interferon therapy, decompensated cirrhosis, concomitant severe illness, proven drug abuse, prothrombin time greater than 4 s prolonged, platelets < 100,000/cmm, WBC < 3000/cmm, granulocytes < 1500/cmm, creatinine > 1.7 mg/dl, anti‐HIV antibodies. |
|
| Interventions | Control: no treatment (n = 30). Experimental 1: interferon alfa‐2b 5 million units (MU) t.i.w. for 4 months, 3 MU thrice a week for 8 months.Duration: 1 year (n = 31). Follow‐up: 1 year post treatment. Liver biopsy at enrolment and second month of post treatment follow‐up. |
|
| Outcomes | Normalisation of ALT. Improvement in liver histology. Decrease in HDAg in liver biopsy. Loss of HDV RNA. | |
| Notes | Drop outs/withdrawals = 13; 5 from the treatment group and 8 from no treatment. Reasons were ulcer at the injection site in1, acute icteric hepatitis in 1, and non‐compliance 3 in the treatment group and 8 in the control group. Intention‐to‐treat analysis done. However, authors preferred per protocol analysis for the histological improvement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Study patients were randomly assigned to the treatment or control group (no placebo) using a computer‐generated randomisation code." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded trial design. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were thirteen (out of 61 patients) dropouts during study period. Quote "Of these 61 patients, 48 (79%) have completed 24 months of the study" |
| Selective reporting (reporting bias) | Low risk | Outcome measures adequately reported. |
| Methods | Sample size: no justification. Generation of allocation schedule: by computer. Allocation concealment: yes. Intention‐to‐treat: yes. Interim analyses: no. | |
| Participants | Patients with chronic hepatitis D (n = 42) from Italy. Inclusion criteria: age 18 to 60, positive HDV antibody, serum HDV RNA documented on three occasions in the last six months, elevated ALT for six months, histological evidence of chronic hepatitis, positive test for intrahepatic delta antigen, no signs of active HBV infection. Exclusion criteria were: antiviral therapy within six months, pregnancy, lactation, decompensated cirrhosis, clotting abnormalities precluding liver biopsy, hepatocellular carcinoma, WBC < 3000/cmm, platelets < 100,000/cmm. |
|
| Interventions | Control: no treatment (n =14). Experimental 1: interferon alfa‐2a 9 million units thrice a week (n =14). Experimental 2:interferon alfa‐2a 3 million units thrice a week (n =14). Duration : 48 weeks. Follow‐up: 6 months post treatment. Long term follow‐up: mean 32 months (24 to 48). |
|
| Outcomes | Loss of HDV RNA. Normalisation of ALT. Improvement in liver histology. | |
| Notes | One patient lost to follow‐up in the control group. Intention to treat analysis was done throughout. Experimental 1 and 2 groups were taken together and compared with the control group. Control group did not get end of treatment biopsy. However, all groups offered six months post treatment biopsy, and these data were used for analysis of histological improvement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | "Using computer‐generated sealed envelopes, we randomly assigned patients..." It does not mention whether the envelops were opaque or not |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded trial design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "All patients with the exception of one in the control group were evaluated at the end of six months of follow‐up." |
| Selective reporting (reporting bias) | Low risk | Outcome measures adequately reported. |
| Methods | Sample size: calculated. Generation of allocation schedule: by computer. Allocation concealment: not used. Intention‐to‐treat analysis: yes. Interim analyses: no. | |
| Participants | Patients with chronic hepatitis D (n =32) from Spain. Inclusion criteria: positive HDV antibody, presence of serum HDV RNA documented in the last six months, elevated ALT for six months, histological evidence of chronic hepatitis or cirrhosis. Six patients (18%) had anti‐HCV and 2 (6%) anti‐HIV antibodies. Exclusion criteria were: antiviral or immunosuppressive therapy within one year, decompensated cirrhosis (Child B or C), concomitant severe illness, proven active drug abuse, prothrombin time less than 50% of normal valve, platelets < 75,000/cmm. | |
| Interventions | Experimental 1: interferon alfa‐2a 18 million units (MU) thrice a week for 6 months, 9 MU thrice a week for 1 month, 6 MU t.i.w. for 1 month, 3 MU thrice a week for 4 months (n =16) . Experimental 2: interferon alfa‐2a 3 million units daily for 3 months then 1.5 MU daily for 9 months (n =16). Duration : 1 year. Follow‐up: 18 months post treatment. Liver biopsy at enrolment and second month of post‐treatment follow‐up. | |
| Outcomes | Loss of HDV RNA. Normalisation of ALT. Improvement in liver histology. | |
| Notes | Drop outs/withdrawals = 7; 3 from low dose and 4 from high dose. Reasons were: return to active drug abuse (n =2), neuropsychiatric disorder (n =2), thrombocytopoenia (n =2), and voluntary withdrawal (n =1). Intention to treat analysis was done throughout. Histological improvement was less than 2 points. No end of treatment biopsy available. Liver biopsy was done after 18 months posttreatment follow‐up period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " The patients were randomly allocated into two groups by means of a computer random‐sample generation." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded trial design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "All but seven (three from group I and four from group II) finished the treatment period... All dropout patients had persistently increased ALT values and HDV RNA positivity when they left the study." Missing outcomes data balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Outcome measures adequately reported. |
| Methods | Sample size: not calculated. Randomisation: performed effectively. Generation of allocation schedule: by computer‐generated randomisation code. Allocation concealment: not used. Intention‐to‐treat: yes. Interim analyses: no. | |
| Participants | Patients with chronic hepatitis D (n =22) from France. Inclusion criteria: age 18 to 65, positive HDV antibody, serum HDV RNA documented in the last six months, elevated ALT (> 1.5 times normal) on three occasions for six months, histological evidence of chronic hepatitis, positive test for intrahepatic delta antigen, no signs of active HBV infection. Exclusion criteria were: antiviral therapy within 24 months, pregnancy, lactation, decompensated cirrhosis, clotting abnormalities precluding liver biopsy, hepatocellular carcinoma, WBC < 3000/cmm, platelets < 100,000/cmm, alcoholism or other drug addiction, or HIV positivity. |
|
| Interventions | Control: no treatment (n =11). Experimental 1: interferon alfa‐2a 5 million units/m2 body surface area thrice a week for 4 months, then 3MU/m2 for 8 months (n =11). Duration : 52 weeks. Follow‐up: 18 months post treatment. | |
| Outcomes | Loss of HDV RNA. Normalisation of ALT. Improvement in liver histology. | |
| Notes | Therapy was discontinued in two patients, one at 4 mo because of induction of hyperthyroidsm and other at 11 months because of death by suicide. Intention to treat analysis was done throughout. Histological improvement was less than 2 points. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, " ... were randomly allocated to receive either no treatment or IFN‐a using a computer generated randomisation code. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible with the trial design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Therapy was discontinued in two patients, one at 4 months because of induction of hyperthyroidsm and other at 11 months because of death by suicide. Intention to treat analysis was done throughout. |
| Selective reporting (reporting bias) | Low risk | Outcome measures adequately reported. |
t.i.w. = three times a week. HDV = hepatitis D virus. anti‐HDV IgM = anti‐hepatitis D virus antibody IgM. HDAg = hepatitis D antigen. ALT = alanine aminotransferase.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Battegay 1994 | Not a randomised clinical trial. |
| Berk 1991 | Not a randomised clinical trial. |
| Borghesio 1995 | Lymphoblastoid IFN 10 MU t.i.w (n = 8) compared with 5 MU daily (n = 6). Both groups treated up to ALT normalization plus 12 months. Basically same dose with two different regimens. No control group. Interm results. |
| Buti 1989 | Not a randomised clinical trial. |
| Canbakan 2006 | Interferon monotherapy compared with interferon plus lamivudine. Though a randomised trial, the trial does not fit within the prespecified comparisons of the review. |
| Castelnau 2006 | Not a randomised clinical trial. |
| Craxi 1990 | Not a randomised clinical trial. |
| Deny 1994 | Not a randomised clinical trial. |
| Di Bisceglie 1990 | Not a randomised clinical trial. |
| Di Marco 1996 | Not a randomised clinical trial. One year treatment compared with two year treatment. Two groups enrolled sequentially; first one year treatment group and then two year treatment group. No control group. Low dose interferon given to both groups. |
| Erhardt 2006 | Not a randomised clinical trial. |
| Farci 1989 | Not a randomised clinical trial. |
| Gunsar 2005 | Interferon monotherapy compared with interferon plus ribavirin. Though a randomised trial, the trial does not fit within the prespecified comparisons of the review. |
| Kaymakoglu 2005 | Not a randomised clinical trial. |
| Lau 1993 | Not a randomised clinical trial. Eleven patients, out of which five treated after one year of observation. Data analysed together. HDV RNA not available. SVR not clear. |
| Lau 1999 | Not a randomised clinical trial. Follow‐up of a single patient. |
| Manesis 2007 | Not a randomised clinical trial. |
| Marinucci 1991 | Not a randomised clinical trial. |
| Marzano 1992 | Not a randomised clinical trial. |
| Puoti 1998 | Not a randomised clinical trial. |
| Rizzetto 1986 | Not a randomised clinical trial. |
| Rumi 1995 A | Not a randomised clinical trial. |
| Rumi 1995 B | Not a randomised clinical trial. |
| Schneieder 1998 | Not a randomised clinical trial. |
| Taillan 1988 | Not a randomised clinical trial. |
| Wedemeyer 2011 | Pegylated interferon monotherapy compared with pegylated interferon plus adefovir and adefovir monotherapy. Though a randomised trial, the trial does not fit within the prespecified comparisons of the review. |
| Wolters 2000 | Not a randomised clinical trial. |
| Yurdaydin 2007 | Not a randomised clinical trial. |
| Yurdaydin 2008 | Interferon monotherapy compared with interferon plus lamivudine and adefovir monotherapy. Though a randomised trial, the trial does not fit within the prespecified comparisons of the review. |
t.i.w. = three times a week. SVR = sustained virological response.
Contributions of authors
Zaigham Abbas drafted the protocol and review, performed the literature search, helped in data extraction, drafted the final review incorporating comments of reviewers. Muhammad Arsalan Khan helped in literature search, performed data extraction, statistical analysis, and co‐drafted the final review. Mohammad Salih helped in literature search for writing the protocol. Wasim Jafri helped in reviewing the studies and revising the final protocol and review.
Sources of support
Internal sources
-
No source utilized, Not specified.
No internal Source of Funding Utilized
External sources
-
No source utilized, Not specified.
No external sources of support utilized
Declarations of interest
None known.
New
References
References to studies included in this review
- Farci P, Mandas A, Coiana A, Lai ME, Desmet V, Eyken PV, et al. Treatment of chronic hepatitis D with interferon alfa‐2A. New England Journal of Medicine 1994;330(2):88‐94. [PUBMED: 8259188] [DOI] [PubMed] [Google Scholar]
- Faure P, Gaudin JL, Naouri C. Treatment of chronic active deltahepatitis with recombinant alpha interferon. The 1990 International Symposium on Viral Hepatitis and Liver Disease April 4–8, Houston. 1990:196. [Google Scholar]; Gaudin JL, Faure P, Godinot H, Trepo C. The French experience of treatment of chronic type D hepatitis with a 12 month course of interferon alpha‐2B. Results of a randomized controlled trial. Liver 1995;15:45‐52. [PUBMED: 7776857] [DOI] [PubMed] [Google Scholar]
- Madejon A, Cotonat T, Bartolome J, Castillo I, Carreno V. Treatment of chronic hepatitis D virus Infection with low and high dose of interferon‐alpha 2a: utility of polymerase chain reaction in monitoring antiviral response. Hepatology 1994;19:1331‐6. [PUBMED: 8188163] [PubMed] [Google Scholar]
- Porres JC, Carreño V, Bartolomé J, Moreno A, Galiana F, Quiroga JA. Treatment of chronic delta infection with recombinant human interferon alpha 2c at high doses. Journal of Hepatology 1989;9(3):338‐44. [PUBMED: PMID: 2691569 ] [DOI] [PubMed] [Google Scholar]
- Rosina F, Saracco G, Lattore V, Quartarone V, Rizzetto M, Verme G, et al. Alpha 2 recombinant interferon in the treatment of chronic hepatitis delta virus (HDV). Progress in Clinical and Biological Research 1987;234:299‐303. [PUBMED: 3628384] [PubMed] [Google Scholar]; Rosina F, Saracco G, Sansalvadore F. Alpha interferon in the treatment of chronic delta hepatitis. Italian Journal of Gastroenterology 1989;21(3):141‐5. [Google Scholar]
- Rosina F, Pintus C, Meschievitz C, Rizzeto M. A randomized controlled trial of a 12‐month course of recombinant human interferon‐alpha in chronic delta (type D) hepatitis: a multicenter Italian study. Hepatology 1991;13:1052‐6. [PUBMED: 2050321] [PubMed] [Google Scholar]; Rosina F, Pintus C, Rizzetto M. Long‐term interferon treatment of chronic hepatitis D: a multicentre Italian study. Journal of Hepatology 1990;11:S149‐S150. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Battegay M, Simpson LH, Hoofnagle JH, Sallie R, Bisceglie AM. Elimination of hepatitis delta virus infection after loss of hepatitis B surface antigen in patients with chronic delta hepatitis. Journal of Medical Virology 1994;44(4):389‐92. [PUBMED: 7897369] [DOI] [PubMed] [Google Scholar]
- Berk L, Man RA, Housset C, Berthelot P, Schalm SW. Alpha lymphoblastoid interferon and acyclovir for chronic hepatitis delta. Progress in Clinical and Biological Research 1991;364:411‐20. [PUBMED: 2020718] [PubMed] [Google Scholar]
- Borghesio E, Rosina F, Marco V, Mangia A, Accogli E, Vandelli C, et al. Long term treatment of chronic hepatitis D with lymphoblastoid interferon: 10 MU vs 5 MU. An interim report of a randomized controlled trial. Hepatology 1995;22(4):397A. [Google Scholar]
- Buti M, Esteban R, Roget M, Jardi R, Allende H, Cascuberta JM, et al. Long term treatment with recombinant alpha 2 interferon in patients with chronic delta hepatitis and human immunodeficiency antibodies. Journal of Hepatology 1989;9:S131. [Google Scholar]
- Canbakan B, Senturk H, Tabak F, Akdogan M, Tahan V, Mert A, et al. Efficacy of interferon alpha‐2b and lamivudine combination treatment in comparison to interferon alpha‐2b alone in chronic delta hepatitis: a randomized trial. Journal of Gastroenterology and Hepatology 2006;21(4):657‐63. [PUBMED: 16677149] [DOI] [PubMed] [Google Scholar]
- Castelnau C, Gal F, Ripault MP, Gordien E, Martinot‐Peignoux M, Boyer N, et al. Efficacy of peginterferon alpha‐2b in chronic hepatitis delta: relevance of quantitative RT‐PCR for follow‐up. Hepatology 2006;44(3):728‐35. [PUBMED: 16941695] [DOI] [PubMed] [Google Scholar]
- Craxi A, Marco V, Volpes R, Marra S, Rizzetto M, Rosina F, et al. Treatment with interferon alpha 2b of chronic HDV hepatitis in children. Journal of Hepatology 1990;11:S175. [Google Scholar]
- Deny P, Fattovich G, Legal F, Giustina G, Lecot C, Morsica G, et al. Polymerase‐chain‐reaction‐based semi‐quantification of hepatitis‐D viremia in patients treated with high‐doses of alpha‐2b interferon. Research in Virology 1994;145(5):287‐95. [PUBMED: 7839006] [DOI] [PubMed] [Google Scholar]
- Bisceglie AM, Martin P, Lisker‐Melman M, Kassianides C, Korenman J, Bergasa NV, et al. Treatment of chronic delta hepatitis with interferon alfa‐2b: pilot study conducted at NIH. Journal of Hepatology 1990;11(Suppl):S151‐S154. [DOI] [PubMed] [Google Scholar]
- Marco V, Giacchino R, Timitilli A, Bortolotti F, Crivellaro C, Calzia R, et al. Long‐term interferon‐alpha treatment of children with chronic hepatitis delta: a multicentre study. Journal of Viral Hepatitis 1996;3(3):123‐8. [PUBMED: 8871870] [DOI] [PubMed] [Google Scholar]
- Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, et al. Treatment of chronic hepatitis delta with pegylated interferon‐alpha2b. Liver International 2006;26(7):805‐10. [PUBMED: PMID: 16911462] [DOI] [PubMed] [Google Scholar]
- Farci P, Karayiannis P, Brook MG, Smedile A, Lai ME, Balestrieri A, et al. Treatment of chronic hepatitis delta virus (HDV) infection with human lymphoblastoid alpha interferon. Quarterly Journal of Medicine 1989;73(271):1045‐54. [PUBMED: 2623137] [PubMed] [Google Scholar]
- Gunsar F, Akarca S, Ersoz G, Kobak AC, Karasu Z, Yuce G, et al. Two‐year interferon therapy with or without ribavirin in chronic delta hepatitis. Antiviral Therapy 2005;10(6):1‐6. [PUBMED: 16218171] [PubMed] [Google Scholar]
- Kaymakoglu S, Karaca C, Demir K, Poturoglu S, Danalioglu A, Badur S, et al. Alpha interferon and ribavirin combination therapy of chronic hepatitis D. Antimicrobial Agents and Chemotherapy 2005;49(3):1135‐8. [PUBMED: 15728914] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, King R, Tibbs CJ, Catterall AP, Smith HM, Portmann BC, et al. Loss of HBsAg with interferon alpha therapy in chronic hepatitis D virus infection. Journal of Medical Virology 1993;39(4):292‐6. [PUBMED: 8492101] [DOI] [PubMed] [Google Scholar]
- Lau DT, Kleiner DE, Park Y, Bisceglie AM, Hoofnagle JH. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology 1999;117(5):1229‐33. [PUBMED: 10535887] [DOI] [PubMed] [Google Scholar]
- Manesis EK, Schina M, Gal F, Agelopoulou O, Papaioannou C, Kalligeros C, et al. Quantitative analysis of hepatitis D virus RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antiviral Therapy 2007;12(3):381‐8. [PUBMED: 17591028] [PubMed] [Google Scholar]
- Marinucci G, Hassan G, Giacomo C, Barlattani A, Costa F, Rasshofer R, et al. Long term treatment of chronic delta hepatitis with alpha recombinant interferon. Progress in Clinical and Biological Research 1991;364:405‐10. [PUBMED: 2020716] [PubMed] [Google Scholar]
- Marzano A, Ottobrelli A, Spezia C, Daziano E, Soranzo ML, Rizzetto M. Treatment of early chronic delta hepatitis with lymphoblastoid alpha interferon: a pilot study. Italian Journal of Gastroenterology 1992;24(3):119‐21. [PUBMED: 1562748] [PubMed] [Google Scholar]
- Puoti M, Rossi S, Forleo MA, Zaltron S, Spinetti A, Putzolu V, et al. Treatment of chronic hepatitis D with interferon alpha‐2b in patients with human immunodeficiency virus infection. Journal of Hepatology 1998;29(1):45‐52. [PUBMED: 9696491] [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Rosina F, Saracco G, Bellando PC, Actis GC, Bonino F, et al. Treatment of chronic delta hepatitis with alpha‐2 recombinant interferon. Journal of Hepatology 1986;3(Suppl 2):S229‐S233. [PUBMED: 3298410] [DOI] [PubMed] [Google Scholar]
- Rumi MG, Ninno E, Rosso R, Parravicini ML, Lampertico P, Soffredini R, et al. Long‐term course of IFN alpha 2b treatment in chronic hepatitis delta. Hepatology 1995;22(Suppl):A183. [Google Scholar]
- Rumi MG, Ninno E, Romeo R, et al. Long‐term course of IFN alpha 2b treatment in chronic hepatitis delta. Abstracts from the Fifth International Symposium on Hepatitis Delta Virus and Liver Disease. Australia: Gold Coast, 1995:D46. [Google Scholar]
- Schneider A, Habermehl P, Gerner P, Lausch E, Ballauff A, Wirth S. Alpha‐interferon treatment in HBeAg positive children with chronic hepatitis B and associated hepatitis D. Klinische Padiatrie 1998;210(5):363‐5. [PUBMED: 9782481] [DOI] [PubMed] [Google Scholar]
- Taillan B, Fuzibet JG, Vinti H, Pesce A, Cassuto JP, Dujardin P. Interferon and delta hepatitis. Annals of Internal Medicine 1988;109(9):760. [PUBMED: 3190061] [DOI] [PubMed] [Google Scholar]
- Wedemeyer H, Yurdaydin C, Dalekos G, Erhardt A, Ya Cakaloglu Y, Degertekin H, , et al. 72 week data of theHIDIT‐1 trial: A multicenter randomised study comparing peginterferon alpha‐2a plus adefovir vs. peginterferon alpha‐2a plus placebo vs. adefovir in chronic delta hepatitis. Journal of Hepatology 2007;46(Suppl):S4. [Google Scholar]; Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. New England Journal of Medicine 2011;364(4):322‐31. [PUBMED: PMID: 21268724] [DOI] [PubMed] [Google Scholar]; Yurdaydin C, Wedemeyer H, Dalekos G, Erhardt A, Cakaloglu Y, Degertekin H. A multicenter randomised study comparing the efficacy of pegylated interferon‐alfa‐2A plus adefovir dipivoxil vs pegylated interferon‐apfa‐2A plus placebo vs adefovir dipivoxil for the treatment of chronic delta hepatitis: the hep‐net/international delta hepatitis intervention trial (HID‐IT). Hepatology 2006;44(4 (Suppl 1)):230A. [Google Scholar]
- Wolters LMM, Nunen AB, Honkoop P, Vossen ACTM, Niesters HGM, Zondervan PE, et al. Lamivudine‐high dose Interferon combination therapy for chronic hepatitis B patients co‐infected with the hepatitis D virus. Journal of Viral Hepatitis 2000;7(6):428‐34. [PUBMED: 11115054] [DOI] [PubMed] [Google Scholar]
- Yurdaydin C, Bozkaya H, Karaaslan H, Onder FO, Erkan OE, Yalçin K, et al. A pilot study of 2 years of interferon treatment in patients with chronic delta hepatitis. Journal of Viral Hepatitis 2007;14(11):812‐6. [PUBMED: 17927618] [DOI] [PubMed] [Google Scholar]
- Yurdaydin C, Bozkava H, Senturk H, Fried MW, Akdogan M, Schinazi FR, et al. Treatment of chronic hepatitis D with lamivudine vs lamivudine+interferon vs interferon: A randomized controlled clinical trial (abstract). Journal of Hepatology 2000;32(2):113. 10728799 [Google Scholar]; Yurdaydin C, Bozkaya H, Onder FO, Sentürk H, Karaaslan H, Akdogan M, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. Journal of Viral Hepatitis 2008;15(4):314‐21. [PUBMED: PMID: 18307594 ] [DOI] [PubMed] [Google Scholar]; Yurdaydin C, Bozkaya H, Onder O, Senturk H, Fried M, Idilman R, et al. Treatment of chronic hepatitis D (CHD) with interferon vs. interferon + lamivudine vs. lamivudine: short‐and long‐term results. Hepatology 2005;42(4):724A‐725A. 16116638 [Google Scholar]
Additional references
- Arrigoni A, Ponzetto A, Actis G, Bonino F. Levamisole and chronic delta hepatitis. Annals of Internal Medicine 1983;98(6):1024. [PUBMED: 6859689] [DOI] [PubMed] [Google Scholar]
- Bordier BB, Ohkanda J, Liu P, Lee SY, Salazar FH, Marion PL, et al. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. Journal of Clinical Investigation 2003;112(3):407‐14. [PUBMED: 12897208] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TZ, Wu JC, Au LC, Choo KB. Specific inhibition of delta antigen by in vitro system by antisense oligodeoxynucleotide: implications for translation mechanism and treatment. Journal of Virological Methods 1997;65(2):183‐9. [PUBMED: 9186941] [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [PUBMED: 3616287] [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. Journal of Hepatology2009; Vol. 50, issue 2:227‐42. [PUBMED: 19054588] [DOI] [PubMed]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P. Delta hepatitis: an update. Journal of Hepatology 2003;39:S212‐S219. [PUBMED: 147087706] [DOI] [PubMed] [Google Scholar]
- Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R, et al. Long‐term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126(7):1740‐9. [PUBMED: 15188169] [DOI] [PubMed] [Google Scholar]
- Farci P, Chessa L, Balestrieri C, Serra G, Lai ME. Traetment of chronic hepatitis D. Journal of Viral Hepatitis 2007;14(Suppl 1):58‐62. [PUBMED: 17958644] [DOI] [PubMed] [Google Scholar]
- Gaeta GB, Stornaiuolo G, Precone DF. Type B and D viral hepatitis: epidemiological changes in Southern Europe. Forum (Geneva) 2001;11(2):126‐33. [PUBMED: 11943358] [PubMed] [Google Scholar]
- Ghany M, Strader D, Thomas D, Seeff L. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009;49:1335‐74. [PUBMED: 19330875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 8. Art. No.: LIVER.
- Hadziyannis SJ. Use of alpha‐interferon in the treatment of chronic delta hepatitis. Journal of Hepatology 1991;13(Suppl 1):S21‐S26. [PUBMED: 1835731] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327:557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
- Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet2011; Vol. 378, issue 9785:73‐85. [PUBMED: 21511329] [DOI] [PubMed]
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
- Ilan Y, Klein A, Taylor J, Tur‐Kaspa R. Resistance of hepatitis delta virus replication to interferon alpha treatment in transfected human cells. Journal of Infectious Diseases 1992;166(5):1164‐6. [PUBMED: 1328404] [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
- Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology 2007;45(2):507‐39. [PUBMED: 17256718] [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Restuccia S, Pistone G, Ruello P, Giugno I, Trovato BA. A meta‐analysis of interferon‐alpha treatment of hepatitis D virus infection. Pharmacotherapy 1996;16:609‐14. [PUBMED: 8840366] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
- Niro GA, Rosina F, Rizzetto M. Treatment of hepatitis D. Journal of Viral Hepatitis 2005;12(1):2‐9. [PUBMED: 15655042] [DOI] [PubMed] [Google Scholar]
- Niro GA, Ciancio A, Tillman HL, Lagget M, Olivero A, Perri F, et al. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized‐controlled pilot study. Alimentary Pharmacology and Therapeutics 2005;22(3):227‐32. [PUBMED: 16091060] [DOI] [PubMed] [Google Scholar]
- Niro GA, Ciancio A, Gaeta GB, Smedile A, Marrone A, Olivero A, et al. Pegylated interferon alpha‐2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006;44(3):713‐20. [PUBMED: 16941685] [DOI] [PubMed] [Google Scholar]
- Ottobrelli A, Marzano A, Smedile A, Recchia S, Salizzoni M, Cornu C, et al. Patterns of hepatitis delta virus reinfection and disease in liver transplantation. Gastroenterology 1991;101(6):1649‐55. [PUBMED: 1955130] [DOI] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Mier W, Lütgehetmann M, Volz T, Weizsäcker F, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nature Biotechnology 2008;26(3):335‐41. [PUBMED: PMID:18297057] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- Rizzetto M, Canese MG, Arico S, Crivelli O, Trepo C, Bonino F, et al. Immunofluorescence detection of new antigen‐antibody system (delta/anti‐delta) associated to hepatitis B virus in liver and serum of HBsAg carriers. Gut 1977;18(12):997‐1003. [PUBMED: 75123] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M, Verme G, Recchia S, Bonino F, Farci P, Arico S, et al. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of delta antigen: an active and progressive disease unresponsive to immunosuppressive treatment. Annals of Internal Medicine 1983;98(4):437‐41. [PUBMED: 6340574] [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Smedile A. Hepatitis D. In: Schiff ER, Sorrell MF, Maddrey WC editor(s). Schiff's Diseases of the Liver. 9th Edition. Philadelphia: Lippincott Williams & Wilkins, 2003:863‐75. [Google Scholar]
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [PUBMED: 15095765] [DOI] [PubMed] [Google Scholar]
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8:79. [PUBMED: 19036172] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
- Sharmeen L, Kuo MY, Dinter‐Gottlieb G, Taylor J. Antigenomic RNA of human hepatitis delta virus can undergo self‐cleavage. Journal of Virology 1988;62(8):2674‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nature Reviews. Gastroenterology & Hepatology 2010;7(1):31‐40. [PUBMED: 20051970] [DOI] [PubMed] [Google Scholar]
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdaydin C, Bozkaya H, Gürel S, Tillmann HL, Aslan N, Okçu‐Heper A, et al. Famciclovir treatment of chronic delta hepatitis. Journal of Hepatology 2002;37(2):266‐71. [PUBMED: 12127433] [DOI] [PubMed] [Google Scholar]


