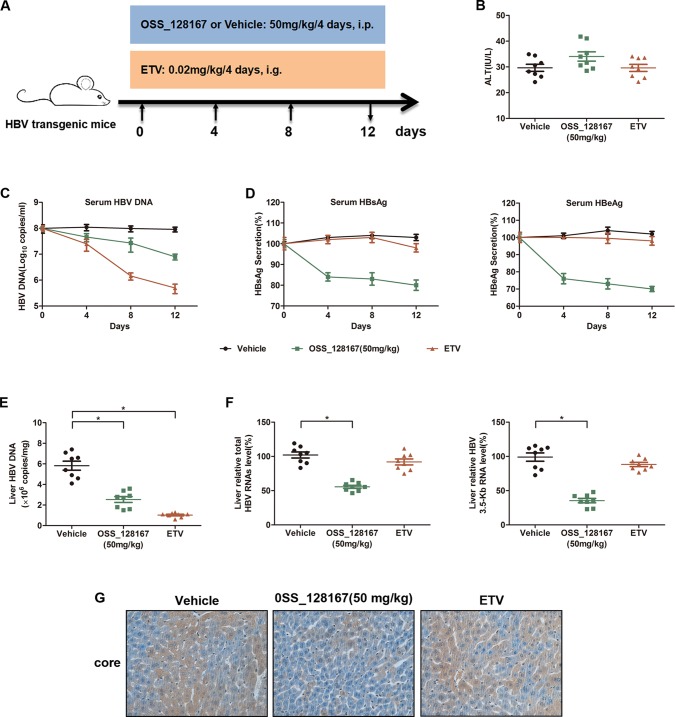
Figure 2.
Antiviral activity of OSS_128167 in a hepatitis B virus (HBV) transgenic mouse model. (A) Workflow of mice model. The mice were randomly divided into three groups (n = 8). The group of OSS_128167 (50 mg/kg) and vehicle were treated every 4 days by intraperitoneal injection. The group of ETV (0.02 mg/kg) was treated every 4 days by oral gavage administration. At the indicated time points, the serum was collected through tail vein. And the mice were euthanized to collect liver tissues at 12 days post-injection. (B) Serum alanine aminotransferase level was determined by colorimetric microplate assay. (C–D) Kinetics of serum hepatitis B surface antigen (HBsAg), hepatitis B envelope antigen (HBeAg), and HBV deoxyribonucleic acid (DNA) were monitored during the treatment. The levels of serum HBsAg and HBeAg were assayed by ELISA. Serum HBV DNA was extracted with Viral Genome DNA/Ribonucleic Acid (RNA) Extraction Kit and determined by using real-time polymerase chain reaction (PCR). (E) Intrahepatic HBV DNA was extracted with Genomic DNA Extraction Kit and detected by using real-time PCR. (F) Liver tissues were harvested to extract total RNAs. Real-time PCR analysis was used to examine total HBV RNAs and 3.5-Kb RNA. β-Actin was used as the internal control. (G) HBcAg in liver tissues was detected by using Immunohistochemical staining. Data represented the mean ± SD of three independent experiments. *:P < 0.05.