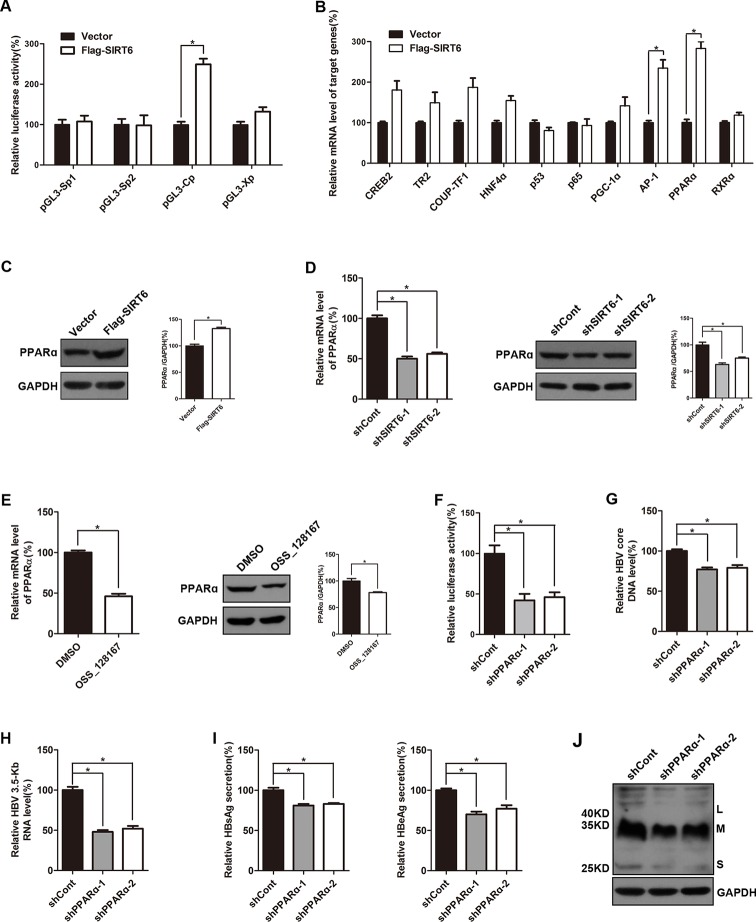
Figure 5.
Sirtuin 6 (SIRT6) enhanced the activity of hepatitis B virus (HBV) core promoter through upregulating transcription factor peroxisome proliferator-activated receptors α (PPARα). (A) Four luciferase reporter constructs were cotransfected with Flag-SIRT6 into Huh7 cells, and the renilla luciferase reporter (RL-TK) was cotransfected to normalized transfection efficiency. The luciferase activities were determined 2 days after transfection. (B) HepG2.2.15 cells were transfected with plasmids expressing Flag-SIRT6. After 3 days post-transfection, total ribonucleic acid (RNA) was extracted by using TRIzol reagent and the expression of various transcription factors associated to HBV transcription were detected by real-time polymerase chain reaction (PCR) with specific primers. β-actin was used as the internal control. (C) HepG2.2.15 cells were transfected with plasmids expressing Flag-SIRT6. Total protein was extracted at 3 days post-transfection and subjected to western blotting. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control. (D) HepG2.2.15 cells were transfected with plasmids expressing shRNAs targeting SIRT6 (shSIRT6-1 and shSIRT6-2) or scramble control short hairpin RNA (shRNA) (shCont). After 3 days post-transfection, total RNA was extracted by using TRIzol reagent and the expression of PPARα was detected by real-time PCR with specific primers. β-actin was used as the internal control. At the same time, total protein was extracted and subjected to western blotting. GAPDH was used as the loading control. Band intensities were quantified by ImageJ software and normalized to GAPDH. (E) HepG2.2.15 cells were treated with OSS_128167 for 3 days. The expression of PPARα was detected by real-time PCR with specific primers. β-Actin was used as the internal control. And total protein was subjected to western blotting. GAPDH was used as the loading control. Band intensities were quantified by ImageJ software and normalized to GAPDH. (F) The Huh7 cells were transfected with pGL3-Cp and RL-TK 24 h after transfection of plasmids expressing PPARα shRNA or scramble control shRNA. The luciferase activity was determined at 72 h posttransfection. RL-TK was used to normalized transfection efficiency. (G–J) HBV-infected HepG2-sodium taurocholate cotransporting polypeptide (NTCP) cells were transfected with plasmids expressing shRNAs targeting PPARα (shPPARα-1 and shPPARα-2) or scramble control shRNA (shCont). (G) HBV core deoxyribonucleic acid (DNA) were extracted at 5 days post-transfection. Then real-time PCR was performed to detect HBV core DNA level. (H) After 4 days post-transfection, total RNA was extracted by using TRIzol reagent and 3.5-Kb RNA level were detected by real-time PCR with specific primers. β-actin was used as the internal control. (I–J) Secretion of hepatitis B surface antigen (HBsAg) and hepatitis B envelope antigen (HBeAg) were assayed by using ELISA 4 days after transfection. At the same time, HBsAg production in cell lysates was determined by western blotting. GAPDH was used as the loading control. Data represented the mean ± SD of three independent experiments. *:P < 0.05.