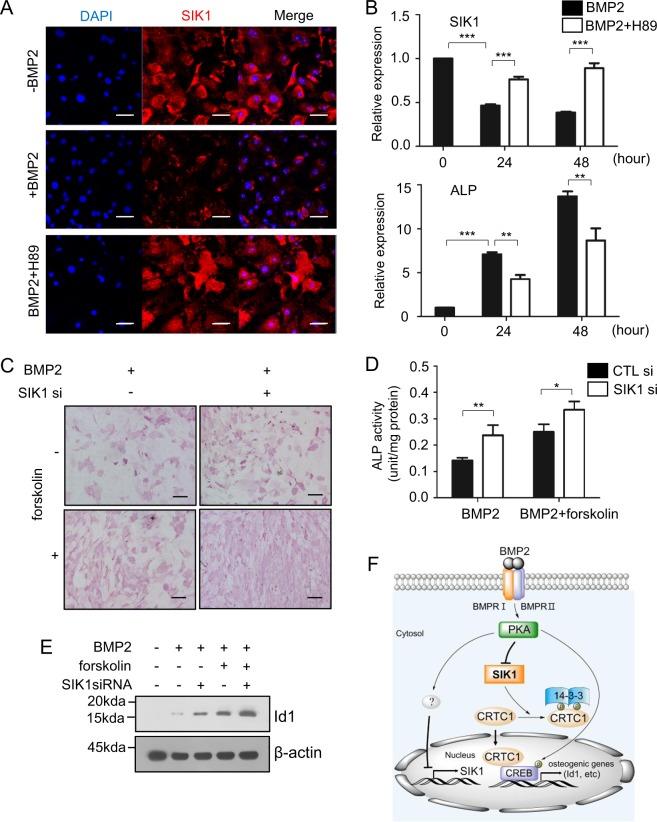
Fig. 7. BMP2 downregulates SIK1 via PKA signaling.
a Primary preosteoblasts were treated with BMP2 (150 ng/mL) for two days in the absence or presence of H89 (10 μM). The protein expression of SIK1 was analyzed by confocal microscopy (Red: SIK1; Blue: DAPI). b Primary preosteoblasts were treated with BMP2 (150 ng/mL) in the absence or presence of H89 (10 μM). The SIK1 and ALP mRNA levels were analyzed by real-time PCR. c, d Primary preosteoblasts transfected with control or SIK1 siRNA were treated with BMP2 (150 ng/mL) for three days in the absence or presence of forskolin (1 μM). Cells were stained for ALP c or cell lysates were subjected to ALP activity assay d. e C2C12 cells were transfected with SIK1 siRNA and stimulated with hBMP2 (150 ng/mL) in the absence or presence of forskolin (10 μM) for 18 h. Id1 protein expression was analyzed by Western blotting, and the level of Id1 was normalized to that of β-actin. ***p < 0.001; **p < 0.01, *p < 0.05. Scale bars, 50 μm a or 200 μm c. f A schematic illustration for the role of SIK1 during osteogenesis. In the absence of BMP2 signaling, SIK1 phosphorylates CRTC1 and thereby inhibits its nuclear translocation, leading to a suppression of CREB target genes. In response to BMP2, SIK1 level is downregulated and SIK1 activity is inhibited by PKA-dependent mechanisms. Under this SIK1-repressed condition, dephosphorylated CRTC1 translocates into the nucleus and stimulates CREB activity for induction of osteogenic genes including Id1