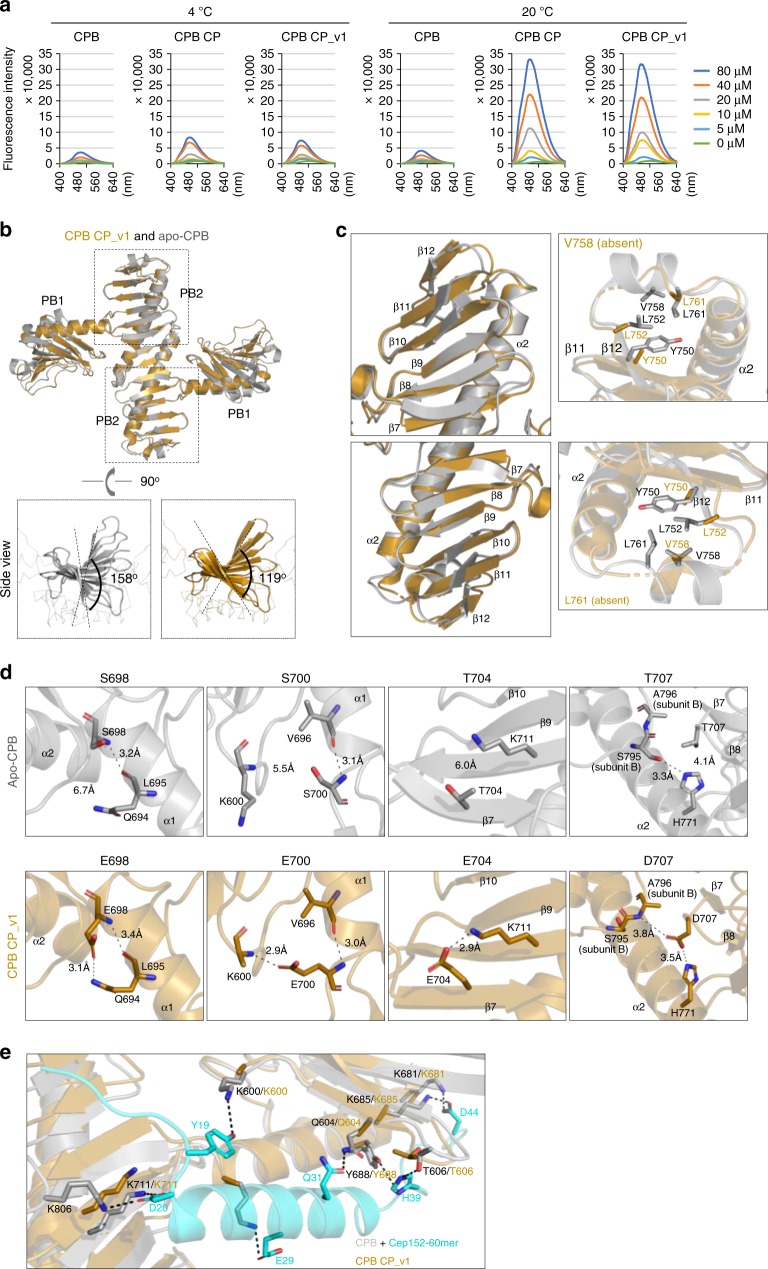
Fig. 5.
Altered surface hydrophobicity and conformational nature of condensation-proficient Plk4 CP and CP_v1. a Fluorescence spectra of ANS in a buffer containing the indicated concentrations of CPB, CPB CP, or CPB CP_v1 at 4 or 20 °C. b Overall structures of the CPB CP_v1 and apo-CPB (4N9J)6 overlaid for comparison. Each subunit of a homodimeric CPB consists of PB1 and PB2 folds. Side views show the magnitude of β-sheet twisting along the PB2–PB2 axis (below). c Detailed structural comparison showing that the PB2-tip region of CPB CP_v1 is angularly distorted (left) and appears flexible with several disordered hydrophobic residues (right). Note that Y750, L752, V758, and L761 residues are either absent or devoid of their side chains. d Differences in the mode of interactions for PC3 WT and CP mutant residues. Important interactions (H-bond and/or salt bridge) are depicted as dashed lines (see text for details). e A hypothetical model illustrating that Cep152 60-mer residues (cyan) engaged in interactions with CPB residues (gray) fail to establish analogous interactions with CPB CP (brown), thus explaining why the CP mutations disrupted the Cep152-Plk4 interaction in Fig. 2d. Dotted lines indicate important interactions detected between Cep152 60-mer and CPB WT (4N7V). Source data are provided as a Source Data file for a