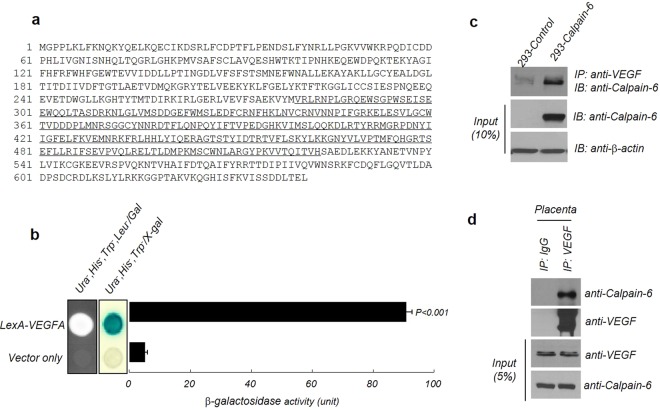
Figure 1.
VEGFA interacts with calpain-6. (a) The amino acid sequence of calpain-6 depicted using single letter abbreviations. The underlined amino acid sequence (279–523) is the translated calpain-6 protein, isolated from the yeast two-hybrid screening analysis. (b) Identification of calpain-6 as a novel VEGFA-interaction protein through yeast two-hybrid screening. To test the interactions between VEGFA and calpain-6, both VEGFA and calpain-6 were expressed as pGilda and pJG4–5 fusion proteins in yeast, and β-galactosidase lift assays were done in the presence of X-gal to assess the binding activity of the constructs. Positive interaction was revealed by cell growth for 3 days at 30 °C on leucine-depleted medium, as well as by the formation of blue colonies on medium containing X-gal. Their interaction was quantitated using the relative activity of β-galactosidase in ONPG assays. B-galactosidase activity was normalized to the value obtained with full-length VEGFA. (c) Co-immunoprecipitation assay for the VEGFA–calpain-6 interaction in HEK293 cells. HEK293 cells were transiently transfected with a calpain-6-overexpressing construct and pcDNA3.1 The Myc-HisA control vector was immunoprecipitated with anti-VEGF, followed by immunoblotting with anti-calpain-6. β-actin was used as an equal loading control. (d) Endogenous calpain-6 and VEGFA interaction in human placental tissue. Placental tissue lysates were prepared in tissue lysate buffer and then subjected to immunoprecipitation with anti-VEGFA antibody, followed by immunoblot analysis with anti-calpain-6.