Table 2:
Investigation of reaction conditions for the preferential formation of the 1,1-arylboration product.[a]
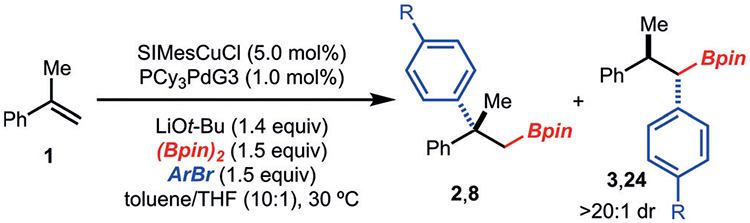 | ||||
|---|---|---|---|---|
| Entry | Change from standard conditions | R | 2,8 [%] | 3,24 [%] |
| 1 | no change | H (2/3) | 3 | 63 |
| 2 | Pi-Bu3PdG3 instead of PCy3PdG3 | H (2/3) | 2 | 39 |
| 3 | Pt-Bu3PdG3 instead of PCy3PdG3 | H (2/3) | 21 | 33 |
| 4 | (PCy3)2Pd instead of PCy3PdG3 | H (2/3) | < 2 | <2 |
| 5 | with 1.0 mol% PCy3 | H (2/3) | < 2 | <2 |
| 6[b] | PdG3 dimer instead of PCy3PdG3 | H (2/3) | 3 | 50 |
| 7[b] | Pd2dba3 instead of PCy3PdG3 | H (2/3) | 3 | 65 |
| 8 | no change | CF3 (8/24) | 4 | 64 |
| 9 | Pd2dba3 instead of PCy3PdG3 | CF3 (8/24) | 2 | 24 |
| 10[b] | PdG3 dimer instead of PCy3PdG3 | CF3 (8/24) | 5 | 42 |
Yields were determined by 1H NMR analysis of the crude reaction mixture with an internal standard.
Pd catalyst loading: 0.5 mol%.
