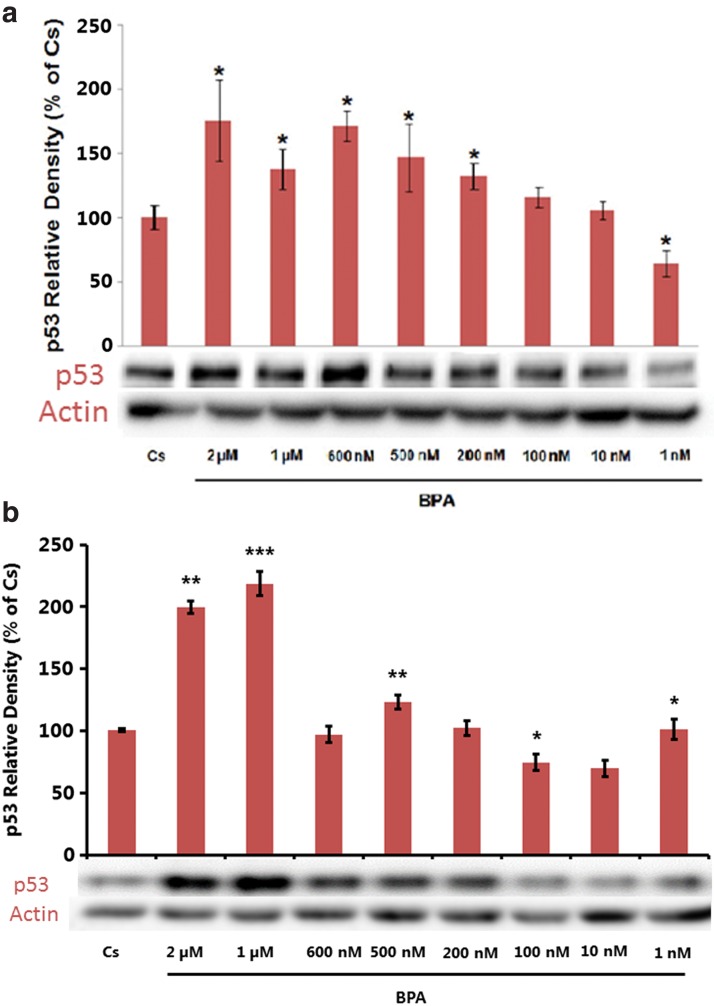
FIG. 1.
(a) T-47D cells were grown for 2 days in RPMI-1640 medium supplemented with 10% FBS and then cultured for 6 days in medium containing 5% DCC-stripped FBS. Semiconfluent cells were then treated with varying concentrations of BPA (1 nM–2 μM). Cellular extracts were prepared and subjected to protein quantification, SDS-PAGE, and Western blot analysis. Lanes labeled Cs represent control lanes with cells grown in the absence of ligands in medium containing 5% SS FBS. Actin bands are shown to indicate equal protein loading among treatment conditions. The relative intensity of p53, compared to Cs, is displayed as the mean ± SEM. The sample sizes came from five density measurements per group. The asterisk indicates significant difference with the control at p < 0.05 (Kruskal–Wallis test followed by post hoc analysis using Mann–Whitney U-test). Representative Western blots are shown. (b) MCF-7 cells were grown for 2 days in Eagle's MEM and supplemented with 10% FBS and then cultured for 6 days in medium containing 5% DCC-stripped FBS. Semiconfluent cells were then treated with varying concentrations of BPA (1 nM–2 μM). Cellular extracts were prepared and subjected to protein quantification, SDS-PAGE, and Western blot analysis. Lanes labeled Cs represent control lanes with cells grown in the absence of ligands in medium containing 5% SS FBS. Actin bands are shown to indicate equal protein loading among treatment conditions. The relative intensity of p53, compared to Cs, is displayed as the mean ± SEM. The sample sizes came from five density measurements per group. The asterisk indicates significant difference with the control at p < 0.05 (Kruskal–Wallis test followed by post hoc analysis using Mann–Whitney U-test). Representative Western blots are shown. BPA, bisphenol A; DCC, dextran-coated charcoal; SS, stripped serum; FBS, fetal bovine serum; MEM, minimum essential medium; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SEM, standard error of the mean.