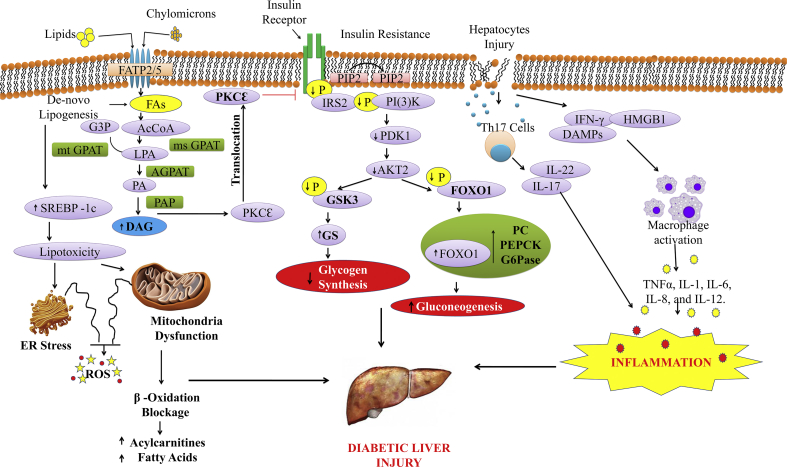
Figure 1.
Molecular mechanisms involved in the pathogenesis of diabetic-associated fatty liver disorders via hepatic insulin resistance and hyperglycaemia. Fatty-acyl coenzymes-A (AcCoAs) (shown here as phosphatidic acid, PA) formed by 1-acylglycerol-3-phosphate O-acyltransferase-2 (AGPAT2) are then added to the glycerol backbone by phosphatidic acid phosphatase (PAP) to generate diacylglycerol (DAG) and by diacylglycerol acyltransferases (DGAT) to generate triacylglycerol (TAG). Increased DAG causes protein kinase Cε (PKCε) translocation to the cell membrane, which inhibits insulin signaling. Reduced phosphorylation of insulin receptor substrate-2 (IRS-2) and PI(3)K impairs AKT2 activity by reductions in 3-phosphoinositide-dependent protein kinase-1 (PDK-1) activity, suppressing glycogen synthase kinase-3 (GSK-3) phosphorylation and reducing insulin-stimulated liver glycogen synthesis through reduced glycogen synthase (GS) activity. Fatty acids (FAs) derived from lipolysis and from chylomicron remnants are taken up through fatty-acid transport proteins (FATPs), mainly FATP2 and FATP5 in the liver. Fatty acids can also be reesterified to lysophosphatidic acid (LPA) by AcCoA and the conversion of glycerol 3-phosphate (G3P) by either mitochondrial glycerol-3-phosphate acyltransferase (mtGPAT) or microsomal GPAT (msGPAT). In case of hepatocytes injury, Th17 cells and DAMPs production lead to the secretion of cytokines and chemokines which cause inflammation. FOXO1, Forkhead box protein O1; PC, pyruvate carboxylase; PEPCK, phosphoenolpyruvate carboxykinase; G6Pase, glucose-6-phosphatase; PIP3, phosphatidyl inositol (3, 4, 5)-triphosphate; Th17, T helper 17 cells; DMPs, damage-associated molecular patterns.