Abstract
Background:
Over the past decades, stroke risk associated with carotid disease has decreased, reflecting improvements in medical therapy and a more rigorous control of vascular risk factors. It is less clear whether the procedural risk of carotid revascularization has declined over time.
Methods and Results:
We analyzed temporal changes in procedural risks among 4,597 patients with symptomatic carotid stenosis treated with carotid artery stenting (CAS; n=2,326) or endarterectomy (CEA; n=2,271) in 4 randomized trials between 2000 and 2008, using generalized linear mixed-effects models with a random intercept for each source trial. Models were additionally adjusted for age and other baseline characteristics predicting treatment risk. The primary outcome event was any procedural stroke or death, occurring during or within 30 days after revascularization. The procedural stroke or death risk decreased significantly over time in all patients (unadjusted OR per year 0.91, 95% CI 0.85–0.97, p=0.006). This effect was driven by a decrease in the CEA group (unadjusted OR per year 0.82, 95% CI 0.73–0.92, p=0.003), whereas no significant decrease was found after CAS (unadjusted OR 0.96, 95% CI 0.88–1.04, p=0.33). CEA patients had a lower procedural stroke or death risk compared to CAS patients, and the difference significantly increased over time (interaction p=0.031). After adjustment for baseline characteristics, the results remained essentially the same.
Conclusions:
The risk of stroke or death associated with carotid endarterectomy for symptomatic carotid stenosis decreased over an 8-year period, independent of clinical predictors of procedural risk. No corresponding reduction in procedural risk was seen in patients treated with stenting.
Clinical Trial Registration:
EVA-3S: https://clinicaltrials.gov/ct2/show/NCT00190398; . SPACE: http://www.isrctn.com/ISRCTN57874028; ISRCTN57874028. ICSS: http://www.isrctn.com/ISRCTN25337470; ISRCTN25337470. CREST: https://clinicaltrials.gov/ct2/show/NCT00004732; .
Keywords: carotid stenosis, stenting, endarterectomy, procedural risk, Cerebrovascular Disease/Stroke, Atherosclerosis, Stenosis, Stent, Revascularization
Graphical Abstract

Introduction
Over the past decades the risk of stroke associated with carotid disease appears to have decreased,1 reflecting improved medical care and risk factor control. In some patients, the risk of stroke under conservative management may be so low that the risks associated with carotid revascularization are no longer justified. This is of relevance for patients with asymptomatic carotid stenosis but potentially also for patients with symptomatic carotid stenosis. On the other hand, the procedural risk associated with carotid revascularization may also have decreased over time preserving the net benefit of invasive treatment. The evidence to support or refute such a trend is currently limited.
We conducted an analysis of the temporal change in procedural stroke or death risks associated with carotid endarterectomy (CEA) and carotid artery stenting (CAS) in the four large randomized controlled trials which enrolled patients with symptomatic carotid stenosis between 2000 and 2008, using data at individual patient level.2–5 We hypothesized that procedural risks associated with carotid artery revascularization would have declined over time. In addition, we assumed that risks of CAS might have decreased more strongly than CEA risks, due to technical development and increasing experience.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This meta-analysis includes individual patient data from EVA-3S (), SPACE (ISRCTN57874028), CREST () and ICSS (ISRCTN25337470).2–5 We excluded trials which included only asymptomatic stenosis, patients deemed at high risk from carotid endarterectomy and those recruiting less than 300 patients. Ethics approval for the contributing trials was obtained at the competent institutional review boards and all patients provided written informed consent. The pooled analysis of individual patient data was agreed upon at the design stage of these trials. The present analysis was pre-specified during one of the regular steering committee meetings of the Carotid Stenosis Trialists’ Collaboration by representatives from the involved trials. All four trials randomly allocated patients with symptomatic moderate to severe carotid stenosis (≥50% reduction of lumen diameter measured according to the method used in the North American Symptomatic Carotid Endarterectomy Trial [NASCET]6), who were equally suitable for either CAS or CEA and considered to be at standard procedural risk. The definition of symptomatic carotid stenosis was symptoms attributable to the relevant carotid artery within 120 days before randomization in EVA-3S, within 180 days before randomization in CREST, within 6 months before randomization in SPACE, and within 12 months before randomization in ICSS. CREST additionally included patients with asymptomatic carotid stenosis, but these patients were excluded from the present analysis.
In EVA-3S, SPACE and ICSS any stent with a CE (Communauté Européeene) mark could be used. In CREST the protocol specified the use of RX Acculink stent. In EVA-3S the use of distal filter protection devices became mandatory early in the trial.7 In CREST, the protocol specified the use of the RX Accunet embolic-protection device whenever feasible. In ICSS and SPACE the use of protection devices remained optional throughout the trials. Surgeons were allowed to perform standard or eversion endarterectomy under local or general anesthesia, with or without the use of shunts or patches.
The primary outcome of the present analysis was any stroke or death occurring within 30 days after treatment. Stroke was defined as an acute deficit of focal neurological function which led to symptoms lasting longer than 24 hours, resulting from intracranial vascular disturbance (ischemia or hemorrhage). Because the trials contributing to this analysis differed in assessment and definition of procedural myocardial infarction, we did not include myocardial infarction in the primary outcome.
Statistical analysis
The analysis population included all patients in whom the randomly allocated treatment was initiated (per-protocol analysis).8 The following baseline characteristics of patients from all 4 source trials were summarized descriptively for an early (2000–2004) and a late enrolment period (2005–2008): sex, patient age, modified Rankin Scale (mRS) at baseline, systolic blood pressure at baseline, history of hypertension, diabetes, lipid-lowering therapy, smoking (past or present), coronary heart disease, degree of ipsilateral carotid stenosis according to NASCET criteria6, presence of contralateral carotid stenosis (≥70%) according to NASCET criteria6 or occlusion, qualifying event (QE) type, and days from QE to treatment.
To investigate whether the risk of stroke or death within 30 days of treatment changed over time, we used generalized linear mixed-effects models (GLMM) with binomial error and logit link function, with a random intercept for each source trial. We fitted one GLMM for all patients, using treatment received (CAS vs. CEA), year of treatment (as continuous variable) and the interaction between treatment received and year of treatment as explanatory variables to investigate if any difference in procedural risk between CAS and CEA changed over time. In addition, a separate GLMM with only year of treatment as explanatory variable was fitted for each treatment group separately. To adjust our models, we identified those baseline patient characteristics which were most strongly associated with procedural risk for stroke or death, using backward model selection based on Akaike’s information criterion (AIC), in all patients and in each treatment group separately. We continued dropping variables from the multivariate models as long as the AIC for the reduced model was smaller than the AIC of the former model. Due to the high percentage of missing values, we did not include days from QE to treatment in the backward model selection. The models investigating the effect of time on procedural risk were subsequently adjusted for all baseline characteristics selected in this manner. We performed a post-hoc sensitivity analysis adjusting all models for days from QE to treatment. We defined an alpha level of 0.05 to ascribe statistical significance. No correction was made for multiple testing. All statistical analyses were performed as complete case analyses (no imputation of missing values), using the statistical software environment R (Version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
In total, 4,775 patients with symptomatic carotid stenosis were enrolled in the contributing trials. The pooled per-protocol analysis set included 4,597 patients, 2,271 of whom received CEA and 2,326 CAS (Figure 1). EVA-3S enrolled patients from 2000–2005, SPACE from 2001–2006, ICSS from 2001–2008, and CREST from 2000–2008. Baseline characteristics were well balanced between treatment groups as previously reported.2–5 The proportions of patients with a history of hypertension, coronary heart disease, smoking and severe ipsilateral carotid stenosis were significantly higher in the later enrolment period (2005–2008) compared to the early enrolment period (2000–2004; Table 1). The proportion of patients taking lipid-lowering therapy significantly increased from 52.4% in the early enrolment period to 74.1% in the late enrolment period. Level of functional disability measured by the mRS was higher and the time from QE to treatment was shorter in the late enrolment period (Table 1).
Figure 1. Study flow chart.
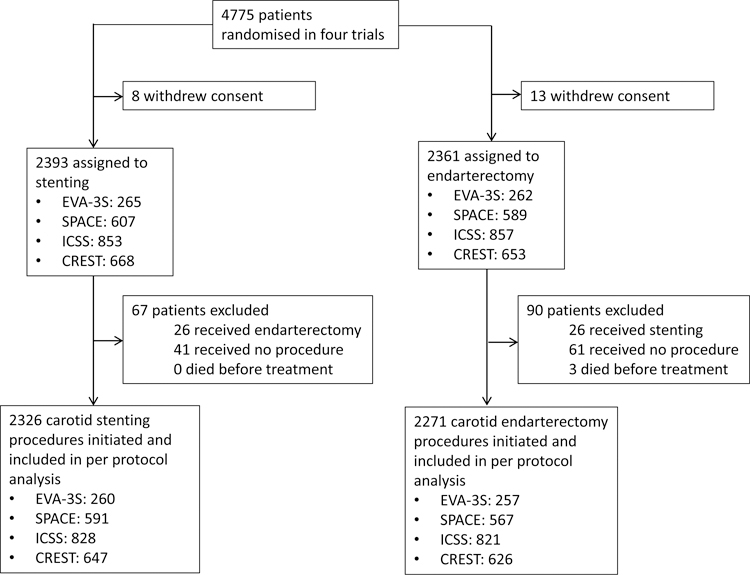
Study flow chart depicting all patients in enrolled in the source trials included in meta-analysis as well as events precluding patients from analysis.
Table 1.
Baseline characteristics in the early (2000–2004) and late (2005–2008) enrolment period.
| 2000–2004 (n = 2,044) | 2005–2008 (n = 2,553) | p-value | |
|---|---|---|---|
| Male sex | 69.8% | 69.8% | 0.995 |
| Age, years (mean, SD) | 69.2 ± 9.4 | 69.3 ± 9.1 | 0.669 |
| Systolic blood pressure at baseline, mmHg (mean, SD) | 143.7 ± 19.8 | 143.1 ± 21.9 | 0.319 |
| Hypertension | 73.8% | 77.0% | 0.016 |
| Diabetes | 25.8% | 24.4% | 0.281 |
| Lipid-lowering therapy* | 52.4% | 74.1% | <0.001 |
| Smoking (current or past) | 62.0% | 67.3% | <0.001 |
| Coronary heart disease | 26.2% | 29.3% | 0.025 |
| mRS at baseline (median [IQR]) | 0 (0–1) | 1 (0–1) | 0.02 |
| Degree of ipsilateral carotid stenosis | <0.001 | ||
| Moderate (50–69%) | 22.4% | 16.7% | |
| Severe (70–99%) | 77.6% | 83.3% | |
| Contralateral stenosis or occlusion | 14.5% | 15.0% | 0.648 |
| Qualifying event type | 0.063 | ||
| Retinal ischemia | 15.7% | 18.3% | |
| Transient ischemic attack | 37.9% | 36.0% | |
| Hemispheric stroke | 46.4% | 45.8% | |
| Days from qualifying event to treatment (median [IQR])† | 32 (15–68) | 26 (11–61) | <0.001 |
Baseline characteristics of patients enrolled in the 4 source trials during the early enrolment period (2000–2004) and the late enrolment period (2005–2008). P-values for differences in baseline characteristics between the early and the late enrolment period were calculated using Welch test for continuous variables, Wilcoxon rank sum test for mRS (not normally distributed), and Chi-squared test for categorical variables. SD indicates standard deviation, mRS: modified Rankin Scale.. EVA-3S recorded lipid-lowering therapy (LLT) use at baseline but patients were only considered to be taking LLT if started >3months prior to randomization. SPACE and CREST collected data on LLT use at randomization. ICSS did not collect information on LLT use at baseline but did collect these data at the one-month follow-up, which were included in the table.
Date of the qualifying event before randomization was not collected in SPACE initially, but for the pooled analysis, these dates were gathered where available. As a result, 16.6% of values for this variable are missing.
Crude percentages of patients with the primary outcome measure per year are shown in Table 2. In the CEA group, crude procedural risks were 7.1% between 2000–2002 and 2.0% between 2007–2008. In the CAS group, crude risks were 8.2% between 2000–2002 and 5.8% between 2007–2008 (Table 2). The risk of stroke or death during the procedural period for both treatments combined decreased significantly over time (unadjusted OR per year 0.91, 95% CI 0.85–0.97, p=0.006). After adjustment for baseline characteristics which were independently associated with the primary outcome in both treatment groups combined (age, mRS, hypertension, diabetes, and severe ipsilateral carotid stenosis; Supplementary table), the decline in risk remained essentially unchanged (adjusted OR 0.89, 95% CI 0.83–0.95, p<0.001). In the post-hoc sensitivity analysis additionally adjusting our models for days from QE to treatment, the results remained again essentially the same (OR 0.91, 95% CI 0.84–0.99, p=0.023).
Table 2.
Procedural risk for stroke or death expressed as crude risks over time
| Year of treatment | All patients (n = 4,597) | Endarterectomy (n = 2,271) | Stenting (n = 2,326) | |||
|---|---|---|---|---|---|---|
| N Patients | N (%) | N Patients | N (%) | N patients | N (%) | |
| 2000–2002 n (%) | 560 | 43 (7.7%) | 280 | 20 (7.1%) | 280 | 23 (8.2%) |
| 2003 n (%) | 659 | 43 (6.5%) | 330 | 18 (5.5%) | 329 | 25 (7.6%) |
| 2004 n (%) | 825 | 37 (4.5%) | 411 | 13 (3.2%) | 414 | 24 (5.8%) |
| 2005 n (%) | 919 | 58 (6.3%) | 439 | 14 (3.2%) | 480 | 44 (9.2%) |
| 2006 n (%) | 630 | 37 (5.9%) | 310 | 13 (4.2%) | 320 | 24 (7.5%) |
| 2007–2008 n (%) | 1004 | 39 (3.9%) | 501 | 10 (2.0%) | 503 | 29 (5.8%) |
| Total | 4597 | 257 (5.6%) | 2271 | 88 (3.9%) | 2326 | 169 (7.3%) |
Total numbers of patients recruited, as well as numbers of patients and crude percentages of patients with the primary outcome measure per year for all patients, patients treated with endarterectomy and patients treated with carotid stenting separately. The years 2000–2003 and 2007–2008 were pooled due to the relatively small number of patients enrolled.
In the CEA group alone, the risk of procedural stroke or death also decreased significantly over time, both in the unadjusted model (OR 0.82, 95% CI 0.73–0.92, p=0.003), in the model adjusted for mRS, hypertension, diabetes, coronary heart disease, ipsilateral severe carotid stenosis, and contralateral stenosis >50% or occlusion ( OR 0.82, 95% CI 0.72–0.93, p=0.002), and in the model additionally adjusted for days from QE to treatment (OR 0.81, 95% CI 0.71–0.93, p=0.005).
In the CAS group alone, the change in procedural risk over time was not statistically significant in the unadjusted model (OR 0.96, 95% CI 0.88–1.05, p=0.33), in the model adjusted for age, hypertension, LLT, smoking, and qualifying event type (OR 0.95, 95% CI 0.87–1.05, p=0.28), nor in the model additionally adjusted for days from QE to treatment (OR 0.95, 95% CI 0.86–1.06, p=0.38).
Patients receiving CEA were at lower risk of procedural stroke or death than patients receiving CAS over the entire enrolment period (OR 0.47, 95% CI 0.35–0.62, adjusted for year of treatment; OR 0.46, 95% CI 0.35–0.62, adjusted for year of treatment, age, mRS at baseline, history of hypertension and diabetes, severe ipsilateral carotid stenosis). This difference in procedural risk became more pronounced over time (unadjusted interaction: OR 1.17, 95% CI 1.02–1.35, p=0.031, adjusted interaction: OR 1.16, 95% CI 1.01–1.34, p=0.038; Figures 2 and 3). The interaction was of similar magnitude but no longer statistically significant when additionally adjusting for days from QE to treatment (OR 1.13, 95% CI 0.96–1.34, p=0.142).
Figure 2. Decline in risk of stroke or death over time – unadjusted model.

Modelled risks of stroke or death occurring within 30 days after treatment over time by treatment group in the unadjusted generalized linear mixed-effects model. Open and closed circles represent fitted values from the model. Error bars represent Bayesian 95% credible intervals. The interaction between type of treatment and year of treatment was statistically significant (interaction p=0.031). CEA – carotid endarterectomy; CAS – carotid artery stenting.
Figure 3. Decline in risk of stroke or death over time – adjusted model.

Modelled risks of stroke or death (fitted values) occurring within 30 days after treatment over time by treatment group in the adjusted generalized linear mixed-effects model. The fitted values are shown for a “model patient” with median age and modified Rankin Scale (mRS) score at baseline, history of hypertension and severe carotid stenosis, but no history of diabetes mellitus. Open and closed circles represent fitted values from the model. Error bars represent Bayesian 95% credible intervals. The interaction between type of treatment and year of treatment was statistically significant (interaction p=0.038). CEA – carotid endarterectomy; CAS – carotid artery stenting.
Discussion
In this meta-analysis of individual patient data from 4 randomized controlled trials, the risk of stroke or death associated with carotid revascularization for symptomatic carotid stenosis decreased significantly over time. When patients were analyzed separately by treatment, the decline in risk over time was only statistically significant in patients treated with carotid endarterectomy. This decrease in risk was independent of clinical risk factors.
Data from the Oxford Vascular Study (Oxvasc) showed a decline in age and sex specific stroke incidence in an unselected population in Oxfordshire, UK between 1981–84 and 2002–04,9 coinciding with a significant increase in the use of blood pressure lowering, antiplatelet and lipid-lowering medication between the two periods. Likewise, meta-regression analyses suggested a decline in annual stroke risk associated with asymptomatic carotid stenosis over the past 20 years.1 Indirect evidence on a decline in stroke risk in patients with symptomatic carotid stenosis can be gathered from TIA registries: the 90-day stroke risk after a TIA caused by large artery atherosclerosis was consistently reported to be around 20% in the last decade, 10, 11 but dropped to merely 6% in a recent publication.12 While some of this decrease may probably have been accounted for by more rapid specialized assessment and early carotid revascularization in selected patients, changes in medical therapy are also likely to be important. A study from Denmark of patients with symptomatic carotid stenosis found a decline in the rate of any recurrent cerebrovascular event prior to carotid revascularization from 29% to 2.5% after introduction of an optimized medical treatment regimen consisting of dual antiplatelet and statin therapy.13
In the original European and North American symptomatic carotid endarterectomy trials establishing the benefit of CEA in patients with symptomatic carotid stenosis, only a minority of patients received statins.14 Since these trials were conducted, medical therapy and risk factor management has improved, not only with more widespread use of statins but also with stricter control of blood pressure and management of other risk factors. A lower stroke risk under conservative management than observed in previous trials may obviate the need for invasive revascularization in many patients with symptomatic or asymptomatic carotid disease. On the other hand, any decline in the procedural risk of stroke or death associated with carotid revascularization would act towards maintaining the net benefit of invasive treatment. Existing literature suggests a decline in procedural risk associated with CEA for asymptomatic carotid stenosis. 15 However, reliable data on procedural risks for symptomatic carotid stenosis have been sparse,16 and it remained unknown if temporal changes differed between CAS and CEA.
Our findings now provide strong evidence for a decline in procedural stroke or death risk associated with revascularization of symptomatic carotid stenosis over time. The availability of data at individual patient level from several randomized clinical trials yielded important strengths. First, we were able to show temporal changes with greater statistical power than was possible at the level of a single trial. Second, we were able to minimize the risk of confounding of the effect of time on procedural risk by a potential change in the characteristics of patients included in the trials during the course of enrolment. Some of the baseline risk factors which were associated with the procedural risk of stroke or death in both treatment groups combined (history of hypertension, disability measured by the mRS, and degree of ipsilateral carotid stenosis) became more prevalent in the later enrolment period. After adjusting for these risk factors, the results remained essentially the same.
A third strength of our study was that we were able to investigate whether any temporal trend in procedural risks would differ between CEA and CAS, owing to the randomized design of the source trials. Wide-spread use of CAS only started a few years before the start of the trials contributing to this meta-analysis. We therefore hypothesized that technical development and increasing experience would lead to a stronger decline in procedural risk with CAS compared to CEA. Surprisingly, we found the opposite to be true. It is possible that investigators became more selective in the patients they included in the trials as enrolment went on, in terms of characteristics that were not measured. If this was the case, any such selection effect must have had a stronger impact on procedural risks of CEA than on risks of CAS. Previous studies suggest that neurophysiological monitoring and intra-operative assessment of the treated carotid artery during the CEA procedure became more frequent over time and that these factors are associated with a lower short-term stroke or death risk.17, 18 It is possible that these factors were also of importance in our study population, but the data were not available for the present analysis. For CAS however, with growing experience, interventionists might have accepted patients in the trials with more difficult anatomy, which may have counteracted any learning-curve effect.
The CREST investigators have previously reported a non-significant decline in the procedural stroke or death risk associated with CAS over time, and an initial decrease followed by an increase in CEA risk for which there was no conclusive explanation.19 Of note, CREST initially included only patients with symptomatic carotid stenosis, but then additionally allowed patients with asymptomatic carotid stenosis in the trial during the course of enrolment. This change in the proportion of the two groups limited the investigation of a temporal trend. The present, pooled analysis, specifically focused on temporal changes in treatment risks in patients with symptomatic carotid stenosis, considered to be at normal surgical risk. Therefore, data of patients with asymptomatic carotid stenosis enrolled in CREST were not incorporated. For the same reason, we did not include data from trials comparing stenting versus endarterectomy in asymptomatic patients only (ACT-1) or in patients considered to be at elevated risk of procedural complications (SAPPHIRE).20, 21 The combined analysis of data from four trials allowed for a more reliable investigation of temporal changes in treatment risks, and whether these differed between CAS and CEA, than was possible at the level of a single trial.
Embolus protection devices (EPD) have been developed to prevent cerebral embolization during the CAS procedure, in an effort to reduce the procedural risk of stroke. Of note, the trials included in the present analysis differed in their policies on EPD use; in the CREST trial, use of a single filter-type device was mandatory. In the EVA-3S, SPACE and ICSS trials combined, EPDs were used in 61% of patients treated with CAS (in 87% of cases, these were filter-type devices)22, but there was no significant difference in procedural stroke risk between patients treated with versus without EPD. However, more recent types of protection devices, exerting arrest or reversal of flow across the stenosis, which may be more effective than filter devices in preventing stroke during CAS,23–26 were not widely used at the time of recruitment in these trials. Therefore our study was not suited to detect an impact of such devices on stroke risk.
The question whether any change in procedural risk of carotid revascularization over time would be explained by an increased use of lipid-lowering therapy was of particular interest. We found an increase of patients taking LLT from 52% in the early enrolment years to 74% in the late enrolment years. However, LLT did not explain procedural risk in the entire study population or in patients treated with CEA. LLT reduced procedural risk in patients treated with CAS but the temporal change in CAS risk was not statistically significant either unadjusted or adjusted for LLT and other risk factors.
As both risk of stroke and procedural risk of revascularization appear to be lower than at the time of the initial CEA trials, substantial uncertainty remains as to which patients will still benefit from carotid revascularization in addition to contemporary medical therapy and risk factor management. Several randomized trials are currently investigating this question, including the Second European Carotid Surgery Trial (ECST-2), the Stent Protected Angioplasty versus Carotid Endarterectomy Trial 2 (SPACE-2), the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2), and the Endarterectomy Combined with Optimal Medical Therapy (OMT) vs OMT Alone in Patients With Asymptomatic Severe Atherosclerotic Carotid Artery Stenosis at Higher-than-average Risk of Ipsilateral Stroke (ACTRIS) trial. Of note, about one in five patients in our study population had moderate degree of stenosis (50–69%). Degree of stenosis is but one of several factors known to predict stroke risk in patients with symptomatic carotid stenosis. Risk models including demographic factors, vascular risk factors, degree of stenosis and plaque morphology help identify patients at high risk of stroke.27 However, the use of such risk models was not specified in any of the contributing trials and the selection of patients requiring revascularization was left to the discretion of the investigators.
Our study has important limitations. First, randomized trials provide data from highly selected centers, operators and patients. Procedural stroke risks may be higher in a “real-world” setting. Thus the external validity of the absolute risks observed in our study is limited. However, as the protocols of the trials remained largely unchanged throughout the studies, we strongly believe that the observed declined in treatment risk is real and externally valid. Second, the trials included in this meta-analysis were conducted between 2000 and 2008. The procedural risk associated with carotid revascularization methods might have declined even further since 2008. Particularly in CAS, the most recent technical developments, such as stent designs with very small open area between struts,28 reverse-flow protection systems,29 and direct trans-cervical access30 were only achieved after completion of the 4 trials included in this meta-analysis and many devices used in these trials are now outdated and now longer in use. Third, in the earliest years of enrolment (2000–2002) most patients included in this analysis were enrolled in either EVA-3S or SPACE. In addition, between 2007 and 2008 enrolment only continued in ICSS and CREST while EVA-3S and SPACE had completed their enrolment. However, the adjustment for source trial included in all of our models should account for any differences between trials. In addition, in a post-hoc analysis in which we excluded all patients from EVA-3S, the results remained essentially unchanged. Fourth, the results obtained in this analysis cannot necessarily be extrapolated to a decline in procedural risk outside of clinical trials. Fourth, due to the high percentage of missing values, we did not include days from QE to treatment in our initial analysis even though this variable was shown to differentially influence the risk of carotid revascularization.31
Conclusions
Treatment of symptomatic carotid stenosis within the examined trials became safer over time. The reduction in stroke or death risk over time was driven by a significant decline in procedural risks in patients treated with endarterectomy. Mechanisms underlying these findings remain to be determined.
Supplementary Material
What is Known:
Over past decades, stroke risk associated with carotid disease has declined, most likely reflecting improvements in medical therapy and better control of vascular risk factors.
Whether the procedural risk associated with revascularization of carotid stenosis has also declined over time, and whether any such temporal trend would differ between carotid endarterectomy and stenting was unclear.
What this Study Adds:
The risk of stroke or death associated with carotid revascularization in clinical trials has decreased over time.
The decline in procedural risk was particularly apparent in patients treated with carotid endarterectomy.
No significant decline in procedural risk was found in carotid artery stenting.
Acknowledgments
Funding
L.H. Bonati was supported by grants from the Swiss National Science Foundation (PBBSB-116873), the University of Basel, Switzerland, and The Stroke Association. M.M. Brown’s Chair in Stroke Medicine at University College London is supported by the Reta Lila Weston Trust for Medical Research. A. Halliday’s research is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Center (BRC). G. Howard is funded by the National Institute of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS).
LHB has received an unrestricted research grant from AstraZeneca, as well as consultancy and advisory board fees from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical. PR has received lecture fees and advisory board fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covedien, and Pfizer.
Footnotes
Disclosures
All other authors declare no competing interests.
References
- 1.Marquardt L, Geraghty OC, Mehta Z and Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010;41:e11–7. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H and Jansen O. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008;7:893–902. [DOI] [PubMed] [Google Scholar]
- 3.Mas JL, Arquizan C, Calvet D, Viguier A, Albucher JF, Piquet P, Garnier P, Viader F, Giroud M, Hosseini H, Hinzelin G, Favrole P, Henon H, Neau JP, Ducrocq X, Padovani R, Milandre L, Rouanet F, Wolff V, Saudeau D, Mahagne MH, Sablot D, Amarenco P, Larrue V, Beyssen B, Leys D, Moulin T, Lievre M, Chatellier G and Investigators E-S. Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke 2014;45:2750–6. [DOI] [PubMed] [Google Scholar]
- 4.Bonati LH, Dobson J, Featherstone RL, Ederle J, Van Der Worp HB, De Borst GJ, Mali WPTM, Beard JD, Cleveland T, Engelter ST, Lyrer PA, Ford GA, Dorman PJ and Brown MM. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The International Carotid Stenting Study (ICSS) randomised trial. Lancet 2015;385:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, Moore WS, Hill MD, Mantese VA, Clark WM, Timaran CH, Heck D, Leimgruber PP, Sheffet AJ, Howard VJ, Chaturvedi S, Lal BK, Voeks JH, Hobson RW 2nd and Investigators C. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med 2016;374:1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991;22:711–20. [DOI] [PubMed] [Google Scholar]
- 7.Mas JL, Chatellier G, Beyssen B and Investigators E-S. Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke 2004;35:e18–20. [DOI] [PubMed] [Google Scholar]
- 8.Carotid Stenting Trialists C, Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, Mali WP, Zeumer H, Brown MM, Mas JL and Ringleb PA. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010;376:1062–73. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, Sandercock PA, Dennis MS, Warlow CP, Bamford JM, Anslow P and Oxford Vascular S. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925–33. [DOI] [PubMed] [Google Scholar]
- 10.Purroy F, Montaner J, Molina CA, Delgado P, Ribo M and Alvarez-Sabin J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke 2007;38:3225–9. [DOI] [PubMed] [Google Scholar]
- 11.Lovett JK, Coull AJ and Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62:569–73. [DOI] [PubMed] [Google Scholar]
- 12.Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhao P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina C, Rothwell PM, Sissani L, Skoloudik D, Steg PG, Touboul PJ, Uchiyama S, Vicaut E, Wong LK and Investigators TIo. One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med 2016;374:1533–42. [DOI] [PubMed] [Google Scholar]
- 13.Shahidi S, Owen-Falkenberg A, Hjerpsted U, Rai A and Ellemann K. Urgent best medical therapy may obviate the need for urgent surgery in patients with symptomatic carotid stenosis. Stroke 2013;44:2220–5. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ and Carotid Endarterectomy Trialists C. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–16. [DOI] [PubMed] [Google Scholar]
- 15.Munster AB, Franchini AJ, Qureshi MI, Thapar A and Davies AH. Temporal trends in safety of carotid endarterectomy in asymptomatic patients: systematic review. Neurology 2015;85:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rerkasem K and Rothwell PM. Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. Eur J Vasc Endovasc Surg 2009;37:504–11. [DOI] [PubMed] [Google Scholar]
- 17.Kallmayer MA, Tsantilas P, Knappich C, Haller B, Storck M, Stadlbauer T, Kuhnl A, Zimmermann A and Eckstein HH. Patient characteristics and outcomes of carotid endarterectomy and carotid artery stenting: analysis of the German mandatory national quality assurance registry - 2003 to 2014. J Cardiovasc Surg (Torino) 2015;56:827–836. [PubMed] [Google Scholar]
- 18.Knappich C, Kuehnl A, Tsantilas P, Schmid S, Breitkreuz T, Kallmayer M, Zimmermann A and Eckstein HH. Intraoperative Completion Studies, Local Anesthesia, and Antiplatelet Medication Are Associated With Lower Risk in Carotid Endarterectomy. Stroke 2017;48:955–962. [DOI] [PubMed] [Google Scholar]
- 19.Howard G, Hopkins LN, Moore WS, Katzen BT, Chakhtoura E, Morrish WF, Ferguson RD, Hye RJ, Shawl FA, Harrigan MR, Voeks JH, Howard VJ, Lal BK, Meschia JF and Brott TG. Temporal Changes in Periprocedural Events in the Carotid Revascularization Endarterectomy Versus Stenting Trial. Stroke 2015;46:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE and Investigators S. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 2008;358:1572–9. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W and Investigators AI. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med 2016;374:1011–20. [DOI] [PubMed] [Google Scholar]
- 22.Wodarg F, Turner EL, Dobson J, Ringleb PA, Mali WP, Fraedrich G, Chatellier G, Bequemin JP, Brown MM, Algra A, Mas JL, Jansen O and Bonati LH. Influence of stent design and use of protection devices on outcome of carotid artery stenting: A pooled analysis of individual patient data. J Neurointerv Surg 2018;10:1149–1154. [DOI] [PubMed] [Google Scholar]
- 23.Leal I, Orgaz A, Flores A, Gil J, Rodriguez R, Peinado J, Criado E and Doblas M. A diffusion-weighted magnetic resonance imaging-based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg 2012;56:1585–90. [DOI] [PubMed] [Google Scholar]
- 24.Castro-Afonso LH, Abud LG, Rolo JG, Santos AC, Oliveira L, Barreira CM, Velasco TR, Pontes-Neto OM and Abud DG. Flow reversal versus filter protection: a pilot carotid artery stenting randomized trial. Circ Cardiovasc Interv 2013;6:552–9. [DOI] [PubMed] [Google Scholar]
- 25.Mokin M, Dumont TM, Chi JM, Mangan CJ, Kass-Hout T, Sorkin GC, Snyder KV, Hopkins LN, Siddiqui AH and Levy EI. Proximal versus distal protection during carotid artery stenting: analysis of the two treatment approaches and associated clinical outcomes. World Neurosurg 2014;81:543–8. [DOI] [PubMed] [Google Scholar]
- 26.Omran J, Mahmud E, White CJ, Aronow HD, Drachman DE, Gray W, Abdullah O, Abu-Fadel M, Firwana B, Mishkel G and Al-Dadah AS. Proximal balloon occlusion versus distal filter protection in carotid artery stenting: A meta-analysis and review of the literature. Catheter Cardiovasc Interv 2017;89:923–931. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA and Warlow CP. Treating individuals 3: from subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet 2005;365:256–65. [DOI] [PubMed] [Google Scholar]
- 28.Park KY, Kim DI, Kim BM, Nam HS, Kim YD, Heo JH and Kim DJ. Incidence of embolism associated with carotid artery stenting: Open-cell versus closed-cell stents. J Neurosurg 2013;119:642–647. [DOI] [PubMed] [Google Scholar]
- 29.Leal I, Orgaz A, Flores A, Gil J, Rodriguez R, Peinado J, Criado E and Doblas M. A diffusion-weighted magnetic resonance imaging-based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg 2012;56:1585–1590. [DOI] [PubMed] [Google Scholar]
- 30.Plessers M, Van Herzeele I, Hemelsoet D, Patel N, Chung EM, Vingerhoets G and Vermassen F. Transcervical Carotid Stenting With Dynamic Flow Reversal Demonstrates Embolization Rates Comparable to Carotid Endarterectomy. J Endovasc Ther 2016;23:249–54. [DOI] [PubMed] [Google Scholar]
- 31.Rantner B, Kollerits B, Roubin GS, Ringleb PA, Jansen O, Howard G and et al. Early endarterectomy carries a lower procedural risk than early stenting in patients with symptomatic stenosis of the internal carotid artery: results from 4 randomized controlled trials. Stroke 2017;48:1580–1587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


