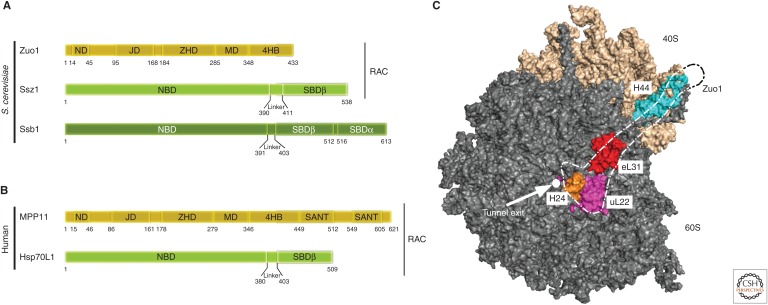
Figure 5.
The ribosome-associated Hsp70/40 system in eukaryotes. (A) The yeast ribosome-associated chaperone system consists of the Hsp40 Zuo1, and the Hsp70s Ssb and Ssz1. Zuo1 and Ssz1 form the stable heterodimeric ribosome-associated complex (RAC). Zuo1 binds to the Ssz1 nucleotide-binding (NBD) and substrate-binding domain β (SBDβ) via its amino-terminal domain (ND). The J domain (JD) of Zuo1 is required for stimulation of the ATPase activity of its Hsp70 partner Ssb. The Zou1 homology domain (ZHD), the highly charged middle domain (MD), and the four-helix bundle (4HB) are involved in ribosome binding. Ssz1 and Ssb contain an amino-terminal NBD and a carboxy-terminal SBD. Ssz1 contains an incomplete SBD, which consists of only the SBDβ moiety, whereas the SBD of Ssb is complete and consists of SBDα and SBDβ. Autonomous ribosome binding of Ssb is mostly mediated via a positively charged stretch at the carboxyl terminus. RAC is conserved in mammals while Ssb homologs are absent (and a soluble Hsp70 is used instead). (B) The human RAC complex is formed by the Hsp40 MPP11 and Hsp70L1. MPP11 contains two SANT domains at its carboxyl terminus. (C) Surface rendering of yeast 60S (gray) and 40S (wheat) subunits with major contact points with Zuo1 highlighted. The dotted empty outline coarsely traces the shape of Zuo1. Exit tunnel (white circle), uL22 (magenta), uL31 (red), helix 24 (H24) of the 25S ribosomal RNA (rRNA) in 60S subunit (orange), and helix 44 (H44) of expansion segment 12 of 18S rRNA in 40S subunit (cyan). S. cerevisiae, Saccharomyces cerevisiae.