Scheme 1.
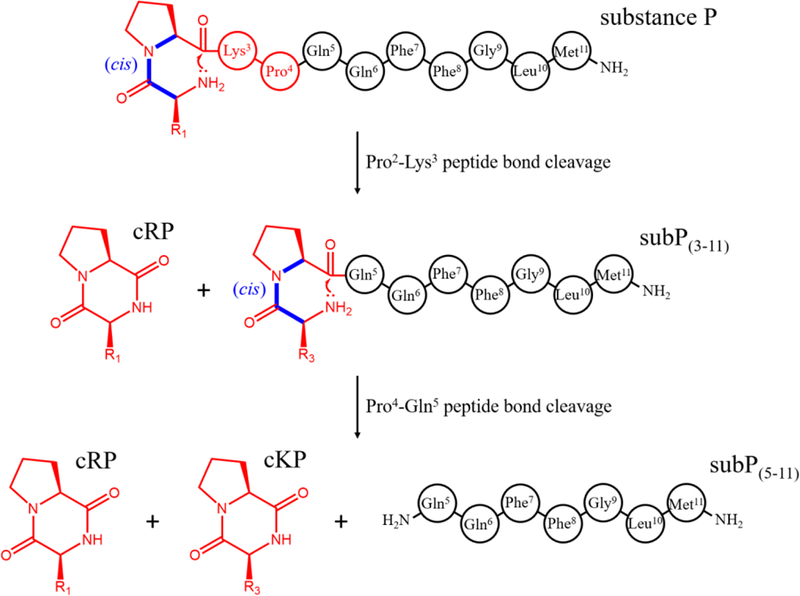
Proposed dissociation pathway of subP. Initially, cleavage occurs at the Pro2-Lys3 bond by attack of the N-terminal amine on the carbonyl carbon of Pro2. This reaction results in a cyclo-Arg1-Pro2 diketopiperazine and the complementary subP(3–11) product. The newly formed subP(3–11) spontaneously cleaves the Pro4-Gln5 bond by the same mechanism to form cyclo-Lys3-Pro4 and subP(5–11).
