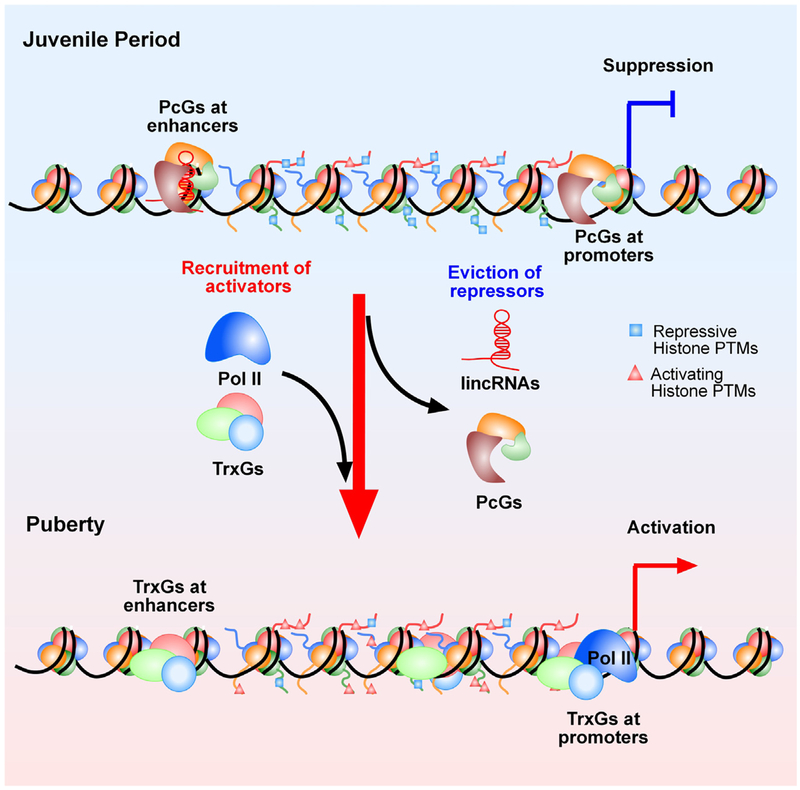
Fig. 5. Postulated epigenetic mechanisms controlling the onset of female puberty.
This model predicts the existence of an antagonistic (Yin-Yang) mechanism of transcriptional regulation underlying the developmental changes in expression of genes that facilitate pubertal development. According to this concept, the transcriptional activity of these genes (Kiss1, Tac2, Nell2, TTF1, others) is repressed during prepubertal development by silencing molecules, such as the PcG complex. PcG proteins catalyze the formation of a repressive chromatin structure characterized by an abundance of histone PTMs associated with gene silencing(such as H3K27me3). As puberty approaches, these “writers” of a repressive chromatin configuration are evicted from, and the content of histone repressive marks is reduced at, promoter regions controlling puberty-activating genes. Along with this change, writers of histone PTMs associated with transcriptional activation, such as H3K4me3 and H3K9, 14ac, are recruited to these regulatory regions resulting in enhanced gene expression. A strong candidate for this activational role is the TrxG activating complex, which antagonizes the silencing effect of PcG by both catalyzing the methylation of histone 3 at lysine 4 (H3K4me3, an activating histone mark) and binding to promoter DNA containing this mark. It is also envisioned that a similar relationship operates in distal enhancers regions controlling puberty-related genes. In this case, PcG deposition of the histone repressive mark H3K27me3, coupled to the presence of H3K4me1 and the absence of Pol II, define the presence of a latent enhancer. This inactive enhancer acquires an active configuration following the implementation of H3K27ac by the TrxG complex, and the recruitment of Pol II in the presence of H3K4me1 (also catalyzed by TrxG).