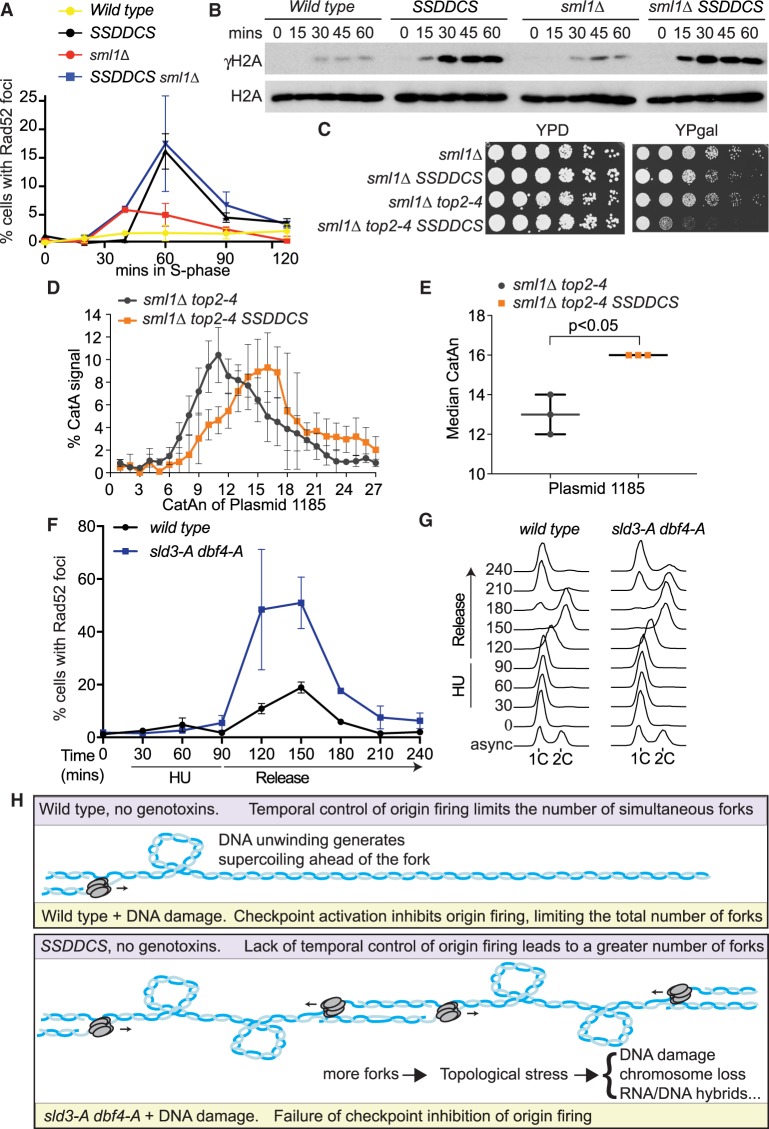
Figure 7.
High rates of replication initiation in a normal S-phase causes similar phenotypes to failure of checkpoint inhibition of origin firing. (A) Quantification of Rad52-GFP foci in the indicated strains released from G1 phase arrest (0 min) into YP galactose medium. The SSDDCS strain expresses limiting replication factors from galactose-inducible promoters. Error bars are SD, n = 3. (B) Western blots of the indicated strains released from G1 phase arrest with alpha factor (0 min) into YP galactose medium. (C) Fivefold dilution growth assays of the indicated strains in the presence (YPgal) or absence (YPD) of expression of SSDDCS. (D) Plot of the distribution of catenated isoforms of the plasmid 1185 as in Figure 5C. Error bars are SD, n = 3. (E) Graph of the median CatAn from D. Error bars are SD, n = 3. (F,G) Quantification of Rad52-GFP foci (F) and flow cytometry (G) of strains released from G1 phase arrest with alpha factor (0 min) into 200 mM HU for 90 min and then washed into HU-free media (release) for a further 150 min. For F, error bars are SD, n = 3. (H) Model for the role of origin firing control in preventing topological stress. Wild-type cells (top) limit simultaneous fork number in a normal S-phase (purple) through a temporal order of origin firing and after DNA damage (yellow) through the checkpoint inhibition of origin firing. In the absence of the checkpoint inhibition of origin firing (sld3-A dbf4-A, yellow, bottom) or in the SSDDCS strain in a normal S-phase (purple, bottom), excess origin firing creates topological problems and increased reliance on pathways to remove supercoils and catenanes. Failure to deal with this stress leads to DNA damage/chromosome loss, possibly through increased RNA/DNA hybrid formation or fork reversal.