Abstract
Chronic sleep restriction, or inadequate sleep, is associated with increased risk of cardiometabolic disease. Laboratory studies demonstrate that sleep restriction causes impaired whole-body insulin sensitivity and glucose disposal. Evidence suggests that inadequate sleep also impairs adipose tissue insulin sensitivity and the NEFA rebound during intravenous glucose tolerance tests, yet no studies have examined the effects of sleep restriction on high-fat meal lipemia. We assessed the effect of 5 h time in bed (TIB) per night for four consecutive nights on postprandial lipemia following a standardized high-fat dinner (HFD). Furthermore, we assessed whether one night of recovery sleep (10 h TIB) was sufficient to restore postprandial metabolism to baseline. We found that postprandial triglyceride (TG) area under the curve was suppressed by sleep restriction (P = 0.01), but returned to baseline values following one night of recovery. Sleep restriction decreased NEFAs throughout the HFD (P = 0.02) and NEFAs remained suppressed in the recovery condition (P = 0.04). Sleep restriction also decreased participant-reported fullness or satiety (P = 0.03), and decreased postprandial interleukin-6 (P < 0.01). Our findings indicate that four nights of 5 h TIB per night impair postprandial lipemia and that one night of recovery sleep may be adequate for recovery of TG metabolism, but not for markers of adipocyte function.
Keywords: triglycerides, nutrition, lipolysis and fatty acid metabolism, diet and dietary lipids, fatty acid, insulin resistance, inflammation, hormones, glucose
According to the Centers for Disease Control and Prevention, one in three US adults sleeps fewer than 7 h per night, increasing their risk of obesity and for risk of developing CVD, type 2 diabetes, and earlier mortality, among other comorbidities (1–4). The mechanisms by which chronic insufficient sleep increases cardiometabolic disease risk are poorly understood, but results from carefully controlled laboratory studies demonstrate that sleep restriction simultaneously increases orexigenic hormonal signaling and impairs glucose metabolic functioning (5, 6). Furthermore, there is mounting evidence that adipocyte insulin sensitivity and function are impaired by sleep restriction resulting in aberrantly elevated overnight and early morning NEFAs (7–10).
Adipocytes are a key integrator of systemic metabolism, absorbing and storing excess energy postprandially and releasing stored fatty acids as needed to meet the energy requirements of the body (11). Adipocytes respond to the postprandial increase in insulin by suppressing intracellular TG lipolysis and by increasing extracellular lipolysis by transporting LPL from intracellular vesicles to the surface of the endothelium (12–14). This results in decreased NEFA release into the plasma and increased absorption of lipoprotein TGs, particularly those in chylomicrons and VLDLs (15–19). Insulin-stimulated adipose tissue biopsies from sleep-restricted subjects have reduced phosphorylation, or activation, of protein kinase B (also called AKT), a protein in the insulin receptor-signaling pathway that is involved in suppression of intracellular lipolysis and NEFA release (7, 13, 20, 21). Sleep restriction increases overnight and early morning NEFA levels, which are correlated with whole-body decreases in insulin sensitivity (8), consistent with the observed impairment of intracellular insulin signaling.
There is also evidence that sleep restriction may alter whole-body fuel selection. Fasting respiratory quotient, a measure of the relative quantity of carbohydrate oxidation to fat oxidation occurring throughout the body, decreases in response to sleep restriction, indicating increased lipid oxidation (9, 22–24). NEFA rebound following the suppression induced by an intravenous glucose tolerance test is delayed and impaired during sleep restriction, consistent with elevated NEFA clearance from the plasma, possibly by skeletal muscle (10). Studies investigating the effects of short-term sleep restriction on circulating lipids have had mixed results. Lipidomic and genomic analyses have found decreased expression of genes involved in cholesterol transport during sleep restriction and decreased circulating choline plasmalogens during total sleep deprivation (25, 26). Choline plasmalogens are a structural component of lipoproteins that have been linked with CVD and are negatively associated with TGs and cardiometabolic risk factors (25). A number of studies have found decreases in fasting TGs with sleep restriction, which is consistent with increased lipid oxidation but at odds with decreased plasmalogens (9, 27, 28). Several other studies found no change in plasma TGs with sleep restriction (29–32). Regardless, evidence from short-term in-laboratory (in-lab) sleep restriction studies is at odds with epidemiological studies, which have consistently found a U-shaped risk of abnormal lipid profiles, including elevated TG, associated with short and long habitual sleep durations (33–36). Research on TG metabolism, particularly postprandial dynamics, may offer a putative link between inadequate sleep and CVD.
Although a large number of studies have examined the effects of sleep restriction on insulin sensitivity and glucose clearance during intravenous glucose tolerance tests and hyperinsulinemic euglycemic clamps, very few studies have examined the effects of sleep restriction on meal digestion and absorption (5, 23, 37, 38). Standardized test meals exploring the effects of sleep restriction on digestion have been served as breakfasts, and most were designed to test postprandial glucose and insulin, with carbohydrates providing the majority of the caloric content (38, 39). Only one sleep restriction study has used a high-fat test meal to assess the thermic effect of food (23). No sleep restriction study has used a high-fat test meal to examine postprandial lipemia, yet evidence from nutrition and epidemiologic studies indicates that postprandial hypertriglyceridemia is strongly related to risk of CVD (40–42). Cardiometabolic risk may also be related to the timing of food intake (43–49). For instance, late-night eating and large dinners are associated with increased BMI (50–53). In weight loss trials where calorie intake is controlled, participants assigned to earlier calorie consumption, versus later, lose more weight (45, 46, 54). Evening lipid digestion may be particularly disturbed during sleep restriction due to elevated evening cortisol; cortisol increases lipolysis, modulates TG synthesis, and decreases insulin-stimulated glucose uptake in adipocytes (8, 39, 55, 56). To date, no studies have examined the effects of sleep restriction on postprandial lipemia, especially the effects of sleep restriction on meal digestion and absorption during the evening hours.
Many Americans eat their largest most calorie-laden meal in the evening and restrict their sleep to meet the demands of the workweek, perform child or elder care, or to meet social obligations, catching up on sleep later (57, 58). We therefore designed this study to examine the effects of sleep restriction [four nights of 5 h time in bed (TIB) per night] and subsequent sleep recovery (one night of 10 h TIB) on postprandial metabolism of a high-fat dinner (HFD). We hypothesized that the reductions in whole-body insulin sensitivity and alterations in lipid oxidation caused by sleep restriction would impair lipid digestion and absorption during an HFD. We further hypothesized that these disturbances would partially or fully recover with one night of recovery sleep. Therefore, we measured postprandial lipemia, glycemia, and the enteric hormonal and inflammatory responses to an HFD during sleep replete, restriction, and recovery conditions.
MATERIALS AND METHODS
All study procedures were reviewed and approved by the Institutional Review Board at the Pennsylvania State University and conducted according to the principles established in the Declaration of Helsinki. Written informed consent procedures are described below.
Recruitment, screening procedures, and consent
Participants were recruited through a mix of online and material advertisements. Interested participants were directed to a secure online screening questionnaire (Qualtrics, Seattle, WA) and study staff followed up with eligible participants. Participants provided written informed consent for screening procedures and then were evaluated based on questionnaire responses and cardiometabolic health as evaluated by a study clinician during a physical examination. Exclusion criteria included: recent travel across time zones, history of shift work, sleep disorders, current or recent medication, tobacco or drug use (confirmed through a urine toxicology screen), and ongoing medical disorders. Exclusion criteria of the physical exam included waist circumference >102 cm, BMI ≤18 kg/m2, seated systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg, HbA1c ≥5.7%, HDL cholesterol <40 mg/dl, LDL cholesterol ≥145 mg/dl, fasting plasma TGs ≥150 mg/dl, and fasting glucose >100 mg/dl. Participants’ habitual sleep was assessed for 1 week via a wrist-worn actigraph (Spectrum; Philips-Respironics, Murrysville, PA) and sleep-wake diary. Finally, participants interviewed with the study psychologist to assess suitability and willingness to participate in the in-lab protocol. Following successful completion of screening procedures, eligible participants met with a senior study investigator and provided written informed consent for the in-lab portion of the study (including pre-study 10 h TIB routine, see below).
Pre-study conditions
Participants maintained a 10 h TIB routine from 2200 to 0800 (±1 h) for at least 1 week (≥6 nights) prior to entering the laboratory. This was triple-confirmed by wrist-worn actigraphy, participant-completed sleep-wake logs, as well as a time-stamped phone log that participants called into each night when they went to bed and each morning when they arose. Participants were instructed to refrain from alcohol, drugs, and caffeine during the pre-study routine. A urine sample was collected from participants at admission for verification of compliance.
In-lab protocol
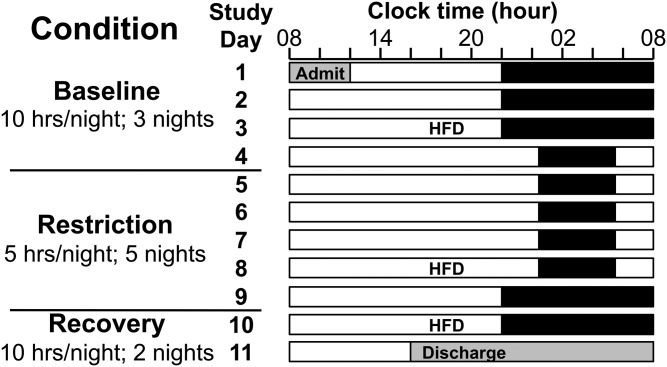
After admission, the 10 h TIB schedule (2200–0800) was maintained for three nights for the baseline condition. For the next five nights, sleep opportunity was limited to 5 h TIB from 0030 to 0530 (sleep restriction condition). Sleep was curtailed evenly, maintaining the nocturnal midpoint, in order to minimize circadian phase shifting and isolate the effects of sleep restriction (independent of circadian misalignment) as much as possible. Finally, the recovery condition consisted of two nights of 10 h TIB resuming the 2200–0800 schedule. See Fig. 1 for a schematic of the study. Participants were put to bed each night and awoken each morning by study staff in order to maintain the scheduled TIB. Study staff monitored participant wakefulness at all times during scheduled wake times (except during showers or restroom use, to maintain participant privacy).
Fig. 1.
Study schematic. Black bars represent TIB. For at least 1 week prior to admission, participants maintained a 10 h TIB routine from 2200 to 0800 (±1 h). Participants were kept on this same TIB schedule for the first three nights in-lab (baseline condition; TIB 2200–0800). For the next five nights, TIB was restricted to 5 h/night, maintaining the nocturnal midpoint (restriction condition; TIB 0030–0530). Participants then resumed the 10 h TIB schedule for two nights (recovery condition; TIB 2200–0800). The HFD was performed after two nights of baseline, four nights of sleep restriction, and one night of recovery sleep (days 3, 8, and 10).
In-lab conditions and environment
Throughout the entire 11 day protocol, participants lived in a private room at the clinical research center. Light levels were controlled [<100 lux in the angle of gaze during wake periods, complete dark (0 lux) during scheduled sleep periods], and temperature was maintained between 20°C and 22°C. Light-emitting devices (i.e., cellphones, computers) were turned off and the participants’ personal electronics were removed from the room each evening 2 h prior to scheduled bed time and were returned no earlier than 2 h after waking. This was done to limit blue-light exposure to study-necessary tasks (i.e., 20 min cognitive tasks performed on an iPad every 2 h), particularly near scheduled sleep periods. Participants were not permitted to sit or recline on the bed during the day except during specific study procedures (see below) and were instructed to remain upright (sitting or standing) throughout scheduled wake periods. Exercise was limited to light stretching.
Controlled diet
The study dietician designed the controlled feeding diet for the in-lab portion of the protocol using weighed foods with predetermined macro- and micronutrient content (Nutrition Data System for Research Software, University of Minnesota, MN). Each day’s diet met the following standards (including on HFD days): 55–60% of calories from carbohydrates, 15–17% of calories from protein, 25–30% of calories from fat, ≥800 mg Ca per day, 130 ± 7 mEq K, and 200 ± 2 mEq Na. All food was prepared in the metabolic kitchen of the clinical research center (Pennsylvania State University, University Park, PA). An average of the Harris-Benedict and Mifflin-St. Jeor equations with low-active activity factors (1.1 and 1.5, respectively) was used to estimate each participant’s total daily energy expenditure and food volumes were adjusted accordingly (59, 60). Participants consumed three meals per day (breakfast, lunch, and dinner) and were instructed to finish each meal within 30 min. Post-void fasted weights were taken each morning to monitor weight stability. Participants consumed exactly the same meals on the day prior to the baseline and sleep restriction standardized HFDs. The prior day’s diet was composed of 59% carbohydrates, 15.1% protein, 25.9% fat, 1,726 mg Ca, 135 mEq K, and 200 mEq Na. Due to the compressed timeframe for recovery procedures, and the need to accommodate an intravenous glucose tolerance test on the morning prior, nutrition on the day before recovery HFD was consumed as part of only two meals (lunch and dinner) and had slightly different micro- and macronutrient content: 56.8% carbohydrates, 15.5% protein, 27.7% fat, 2,056 mg Ca, 129 mEq K, and 201 mEq Na.
Actigraphic and polysomnographic sleep quantitation
Participant sleep at home prior to admission (both habitual and the pre-study 10 h TIB routine) was assessed via actigraphy (Spectrum; Philips-Respironics, Murrysville, PA). In-lab sleep was assessed through both actigraphy and polysomnography (PSG) (Nihon Kohden, Irvine, CA). Actigraphy recordings were assessed for participant compliance (wear/non-wear status) and data quality and were double-scored by trained condition-blinded research technicians. Discrepancies between scorers (if any) >15 min were rectified prior to unblinding; device recordings of activity counts, light levels, and wear/non-wear status were used to aid scoring. Actigraphy scoring methods have previously been described (10, 61). A registered polysomnographic technologist staged the in-lab PSG recordings in 30 s windows according to American Academy of Sleep Medicine standards (62, 63). Nights with ≥5.5% unscorable data in the sleep opportunity window (from lights out to lights on), due to calibration, disconnection, or other artifact, were excluded from the analyses. All participants retained at least one night of data in each of the baseline, restriction, and recovery conditions, and therefore none were excluded from sleep analyses. Lights off and lights on times were recorded by study staff; if logged times were inconsistent with PSG data, actigraph light data provided clarification. The time between verified lights off/on and the beginning/end of recorded data was interpolated as “awake” and was included in the sleep opportunity time or TIB.
Standardized HFD
Participants consumed a standardized HFD (1,041 kcal: 48.9 g of fat, 110.5 g of carbohydrate, 46.6 g of protein) during each condition of the study (baseline, sleep restriction, recovery) on days 3, 8, and 10 (see Fig. 1). Breakfast and lunch prior to the HFD were lower in fat and calories such that overall daily calories and macro- and micronutrient targets matched all other study days (see Controlled diet above). Participants finished eating lunch on HFD days no later than 1230 to allow for a minimum of 4 h without food prior to the start of the HFD. An intravenous line was placed in the participant’s forearm at approximately 1600 (45 min prior to meal start time). Participants remained in a reclined position during intravenous line insertion and the first round of baseline blood draws to ensure intravenous line function, after which they were allowed to resume activities at their desks. Following baseline blood draws, the HFD was served at 1645. Participants were instructed to consume the entire meal within 20 min of taking their first bite. Blood samples were drawn every 10 min for the first hour (relative to meal start time) and every 30 min thereafter for 4 h. Blood was collected into 3% EDTA tubes (BD Vacutainers; Becton, Dickinson and Co., Franklin Lanes, NJ) and immediately processed. Enzyme inhibitors (aprotinin and DPP-IV inhibitor) were added to a portion of each blood sample. Samples were spun at 3,000 relative centrifugal force for 10 min and then held at 4°C until aliquoted. Aliquoted samples were maintained at −80°C until analysis. Prior to meal start and immediately after finishing the meal, participants completed hunger and satiety questionnaires using visual analog scales. Participants reported their current hunger and feeling of fullness on visual analog scales from not at all hungry or not at all full to extremely hungry or extremely full, respectively. Responses were scored from 0 to 100 as previously described (64).
Assays
Glucose and TGs were quantified using glucose hexokinase-linked and glycerol phosphate oxidase-linked colorimetric assays (Fisher Diagnostics, Middletown, VA). NEFAs were quantified using an acyl-CoA synthetase-linked colorimetric assay (FUJIFILM Wako Diagnostics, Mountain View, CA). C-peptide, ghrelin, glucagon-like peptide-1 (GLP-1) (active), glucagon, interleukin-6 (IL-6), insulin, leptin, and monocyte chemoattractant protein-1 (MCP-1) were quantified by fluorescent microbead multiplex assay (Eve Technologies Corporation, Calgary, Alberta, Canada). Samples were run in triplicate on colorimetric assays and singlicate on multiplex assays. All plates included a standard curve and two human plasma controls. Multiplex plates also used a validated standard plasma matrix for background wells. Inter-assay variability was monitored and plates were repeated if >5%.
Statistical analyses
Mixed models with random effects for individuals were used for summary data indicators (i.e., areas under the curve (AUCs), comparison of baseline means, sleep means; SAS 9.4M6, Cary, NC). Analyte time-courses were analyzed in mixed models with random effects for individuals and were modeled as a function of condition (baseline, restriction, recovery), time (time into procedure relative to the start of HFD consumption), and the interaction of condition × time. Time was included up to the second polynomial. Sampling times during the procedure were unequally spaced so covariance structure was modeled using the spatial power structure for each combination of subject and condition. Residuals were tested for normality; ghrelin, GLP-1, MCP-1, NEFAs, and TGs were log-transformed. Two subjects were excluded from IL-6 analyses due to missing data (75% and 47% of samples, respectively, had IL-6 values below the threshold of detection). A median-split was performed on IL-6; the data were then assessed in a mixed effect model (as described above) assuming a binary distribution (SAS 9.4M6, Cary, NC). TG population clearance was calculated by deriving the slope from the average of the final four time points (minutes 210–300), extrapolating to baseline for each condition, calculating total TG AUC, and then dividing the dose of meal lipids by total TG AUC (GraphPad Prism 8.0.2, San Diego, CA). Values are reported as significant if the P-value is <0.05. BMI was tested as a covariate but was not significant for any metabolite, so it was removed from the final models.
RESULTS
Participants
Fifteen healthy men (mean ± SD: age 22.33 ± 2.82 years; BMI 24.69 ± 2.99 kg/m2) completed this study. The ethnic/racial composition of the sample was 60% (n = 9) non-Hispanic white, 20% (n = 3) non-Hispanic black, and 20% (n = 3) Asian. Participant weights increased slightly (average increase of 0.37 kg) with four nights of sleep restriction compared with baseline (P = 0.03). Participant weights following one night of recovery sleep were not significantly different from baseline (P = 0.98).
Actigraphic and polysomnographic measures of sleep duration
We have previously reported the actigraphy-quantified sleep in a subset of participants from this study (10). Actigraphy-assessed habitual sleep was 8.4 ± 0.1 h/night (mean ± SEM). Participants significantly increased their sleep during the pre-study 10 h TIB routine to 9.1 ± 0.1 h/night (P < 0.01). There was a further increase in actigraphy-assessed sleep during the two nights of baseline to 9.7 ± 0.2 h/night (P = 0.03). Compared with baseline, participants slept significantly less during the four nights of sleep restriction (4.8 ± 0.2 h/night; P < 0.01). Actigraphy-assessed sleep during one night of recovery was not different from baseline sleep duration (9.7 ± 0.3 h/night; P = 0.98).
PSG-assessed total sleep time, which subtracts epochs of wake, in the two nights of baseline sleep was 8.64 ± 0.1 h/night. As designed, sleep was significantly decreased in the sleep restriction condition compared with baseline (4.74 ± 0.08 h/night; P < 0.01). PSG-assessed sleep during the one night of recovery was increased compared with baseline (9.30 ± 0.13 h/night; P < 0.01).
Glucose and pancreatic analytes
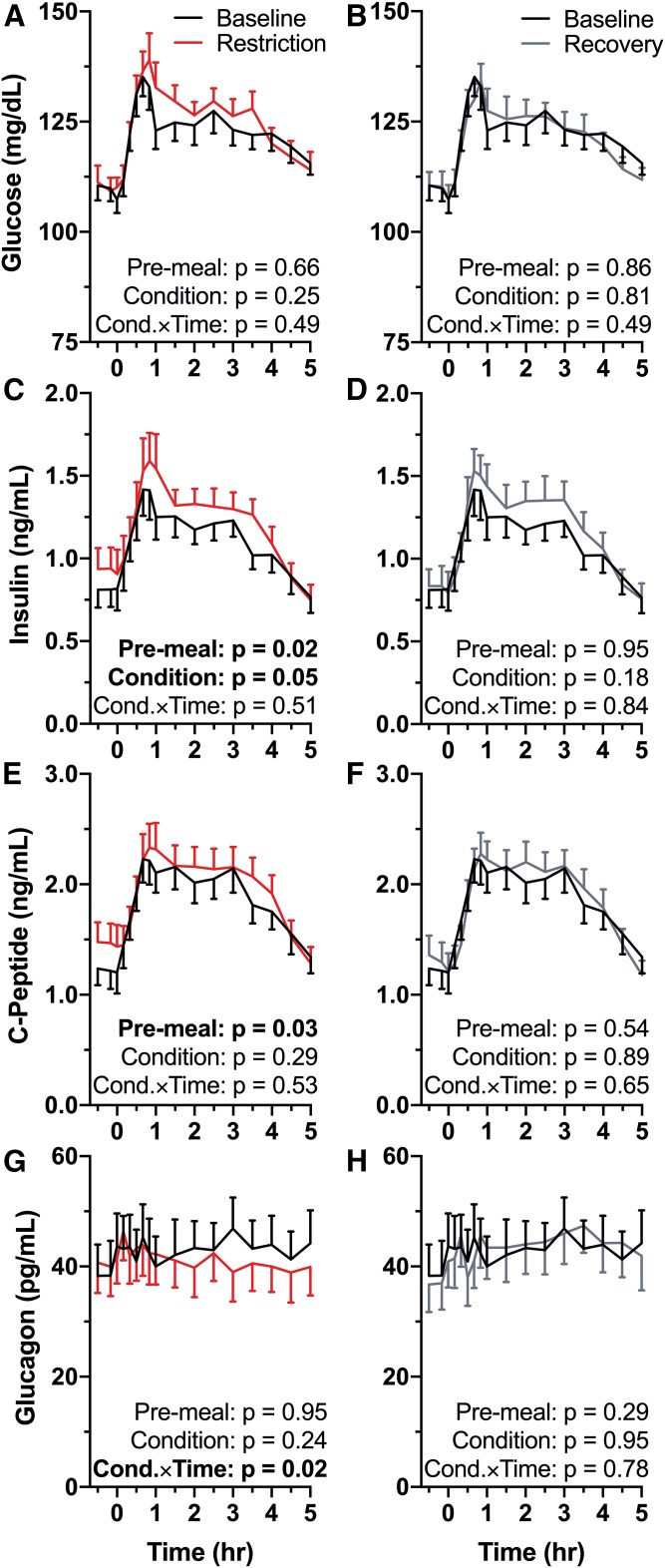
There were no differences in pre-meal glucose (minutes −30 to 0) or glucose AUC (minutes 0–300) during sleep restriction compared with baseline (P = 0.66 and P = 0.85, respectively, Fig. 2A), or between baseline and recovery conditions (pre-meal P = 0.86; AUC P = 0.42, Fig. 2B). Glucose across the entire HFD time course was not significantly different between baseline and sleep restriction (condition P = 0.25; condition × time P = 0.49; Fig. 2A), or between baseline and recovery (condition P = 0.81; condition × time P = 0.49; Fig. 2B).
Fig. 2.
Effects of sleep restriction on postprandial glycemic and pancreatic analytes. Mean values are plotted ± SEM. P-values are reported on each graph (n = 15) and significant P-values (<0.05) are in bold type. Pre-meal = differences during minutes −30 to 0, Condition = significance of the sleep condition effect, Cond.×Time = significance of the condition × time interaction term. Graphs are oriented relative to meal start time (first bite = time 0). Black symbols, baseline (10 h TIB per night for two nights); red symbols, sleep restriction (5 h TIB per night for four nights); gray symbols, recovery (10 h TIB per night for one night). A: Baseline versus restriction HFD glucose. B: Baseline versus recovery HFD glucose. C: Baseline versus restriction HFD insulin. Pre-meal insulin was significantly elevated compared with baseline (P = 0.02). Insulin was elevated throughout the HFD by sleep restriction (condition P = 0.05). D: Baseline versus recovery HFD insulin. E: Baseline versus restriction HFD c-peptide. Pre-meal c-peptide was elevated in the sleep restriction condition (P = 0.03). F: Baseline versus recovery HFD c-peptide. G: Baseline versus restriction HFD glucagon. Glucagon response to the HFD across time differed between baseline and restriction (condition × time interaction P = 0.02). H: Baseline versus recovery HFD glucagon.
Despite no differences in pre-meal glucose values, pre-meal insulin was significantly increased during sleep restriction compared with baseline (P = 0.02; Fig. 2C). In our model, there was a marginally significant effect of the restriction condition compared with baseline (P = 0.05) with no significant condition × time interaction (P = 0.51). The elevated pre-meal insulin levels returned to baseline values with one night of recovery sleep (P = 0.95; Fig. 2D). Across the entire HFD response, there was no significant effect of condition on insulin in recovery compared with baseline (P = 0.18). The condition × time interaction term was also not significant (P = 0.84).
Pre-meal c-peptide was increased in the restriction condition compared with baseline (P = 0.03; Fig. 2E). There was no significant effect of condition between baseline and restriction (P = 0.28) and no significant effect of condition × time (P = 0.53). In the recovery condition, pre-meal c-peptide was not different from baseline (P = 0.54; Fig. 2F). Across the entire HFD there was no difference in c-peptide between baseline and recovery conditions (condition P = 0.89; condition × time P = 0.65).
Neither pre-meal glucagon nor the main effect of condition was different between baseline and sleep restriction (pre-meal P = 0.95; condition P = 0.24; Fig. 2G). There was a significant condition × time interaction, indicating that glucagon decreased across time during sleep restriction compared with baseline (P = 0.02). Between the baseline and recovery conditions, there was no significant difference in pre-meal glucagon (P = 0.29), in the main effect of condition (P = 0.95), or in the interaction of condition × time (P = 0.78; Fig. 2H).
Hunger and satiety hormones and incretins
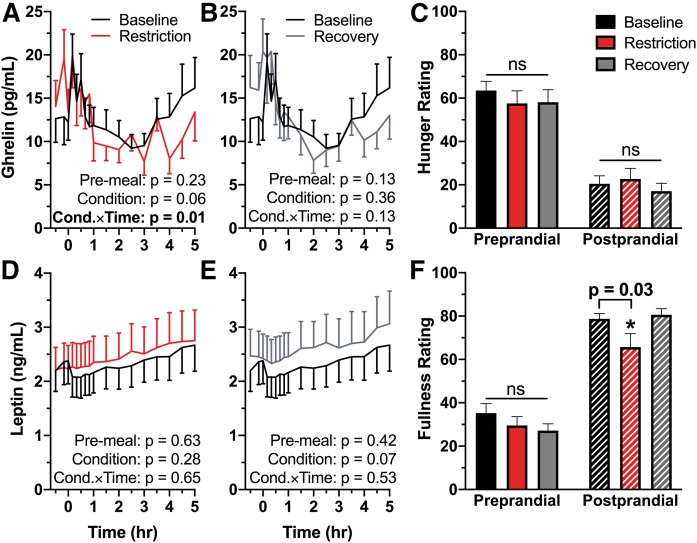
Pre-meal ghrelin was not different in sleep restriction compared with baseline (P = 0.23; Fig. 3A). While there was no effect of condition as a whole (P = 0.06), there was a significant condition × time interaction in ghrelin between baseline and sleep restriction (P = 0.01). There was no difference in self-reported hunger prior to the start of the procedure (P = 0.24) or following meal completion (P = 0.47; Fig. 3C). In the recovery condition, there was no difference in pre-meal ghrelin (P = 0.13), no significant effect of condition (P = 0.36), nor a significant condition × time interaction (P = 0.13; Fig. 3B). There was no difference between the baseline and recovery conditions in self-reported hunger prior to the start of the HFD (P = 0.29) nor following meal completion (P = 0.28; Fig. 3C).
Fig. 3.
Effect of sleep restriction on pre- and postprandial hunger and satiety hormones and perception. Mean values are plotted ± SEM (n = 15). Significant P-values (<0.05) are in bold type. Line graphs are oriented relative to meal start time (first bite = time 0). A: Baseline versus restriction HFD ghrelin. Ghrelin response to the HFD across time differed between baseline and restriction [condition × time (Cond.×Time) interaction P = 0.01]. B: Baseline versus recovery HFD ghrelin. C: Participant-reported hunger ratings immediately prior to the meal and immediately following meal completion. D: Baseline versus restriction HFD leptin. E: Baseline versus recovery HFD leptin. F: Participant-reported fullness ratings immediately prior to and following the HFD. Participants reported feeling less full following the meal in the sleep restriction condition compared with baseline (P = 0.03).
Pre-meal leptin was not different from baseline in the sleep restriction condition (P = 0.63), nor was there an effect of condition (P = 0.28) or a condition × time interaction (P = 0.65; Fig. 3D). Participant self-reported fullness following the meal was significantly decreased in sleep restriction (P = 0.03; Fig. 3F). Similarly, there were no differences in pre-meal leptin (P = 0.42), an effect of condition (P = 0.07), or a condition × time interaction (P = 0.53; Fig. 3E) between the baseline and recovery conditions. Satiety ratings following the meal were not different from baseline in the recovery condition (P = 0.74; Fig. 3F).
Between baseline and sleep restriction there was no difference in pre-meal GLP-1 (P = 0.19), no effect of condition (P = 0.73), and no condition × time interaction (P = 0.41). There were also no differences in pre-meal GLP-1 (P = 0.88) in the recovery condition compared with baseline, nor were there effects of condition (P = 0.87) or a condition × time interaction (P = 0.65).
Inflammatory markers
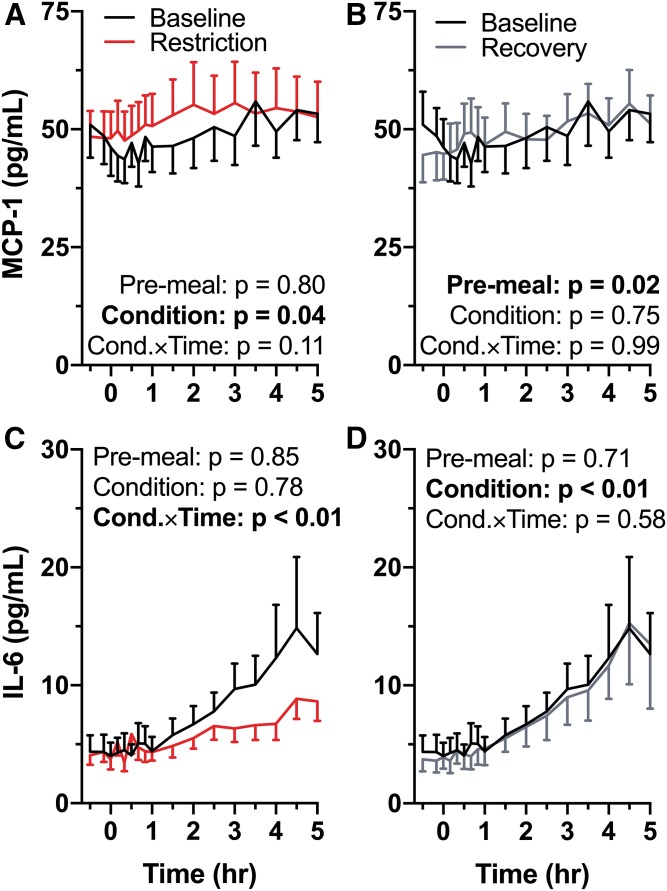
During sleep restriction, MCP-1 pre-meal levels were not different than baseline (P = 0.80; Fig. 4A). Across the entire HFD, there was a significant effect of restriction condition compared with baseline (P = 0.04), but no condition × time interaction (P = 0.11). Pre-meal MCP-1 levels were decreased in the recovery condition compared with baseline (P = 0.02), but there was no significant effect of recovery condition (P = 0.75) or condition × time interaction (P = 0.99; Fig. 4B) across the entire HFD.
Fig. 4.
Effect of sleep restriction on postprandial inflammatory markers. Mean values are plotted ± SEM [n = 15 (A, B); n = 13 (C, D)]. Significant P-values are in bold type. Graphs are oriented relative to meal start time (first bite = time 0). A: Baseline versus restriction MCP-1 response to the HFD. Sleep restriction increased MCP-1 across the entirety of the meal (P = 0.04). B: Baseline versus recovery HFD MCP-1. Pre-meal MCP-1 was decreased in the recovery condition (P = 0.02). C: Baseline versus restriction HFD IL-6. During sleep restriction IL-6 did not increase postprandially to the same degree as at baseline [condition × time (Cond.×Time) interaction; P < 0.01]. D: Baseline versus recovery HFD IL-6. When a median split is applied (necessary due to exponential nature of inflammatory responses), IL-6 is less likely to be elevated during recovery compared with baseline (condition effect; P < 0.01).
There was no difference in pre-meal IL-6 between baseline and restriction (P = 0.85; Fig. 4C). There was also no significant effect of condition (P = 0.78), but there was a significant interaction of condition × time (P < 0.01) during sleep restriction compared with baseline. Pre-meal IL-6 during recovery was not significantly different than baseline (P = 0.71; Fig. 4D). There was a significant effect of condition (P < 0.01), but no interaction of condition × time (P = 0.58).
Lipemia
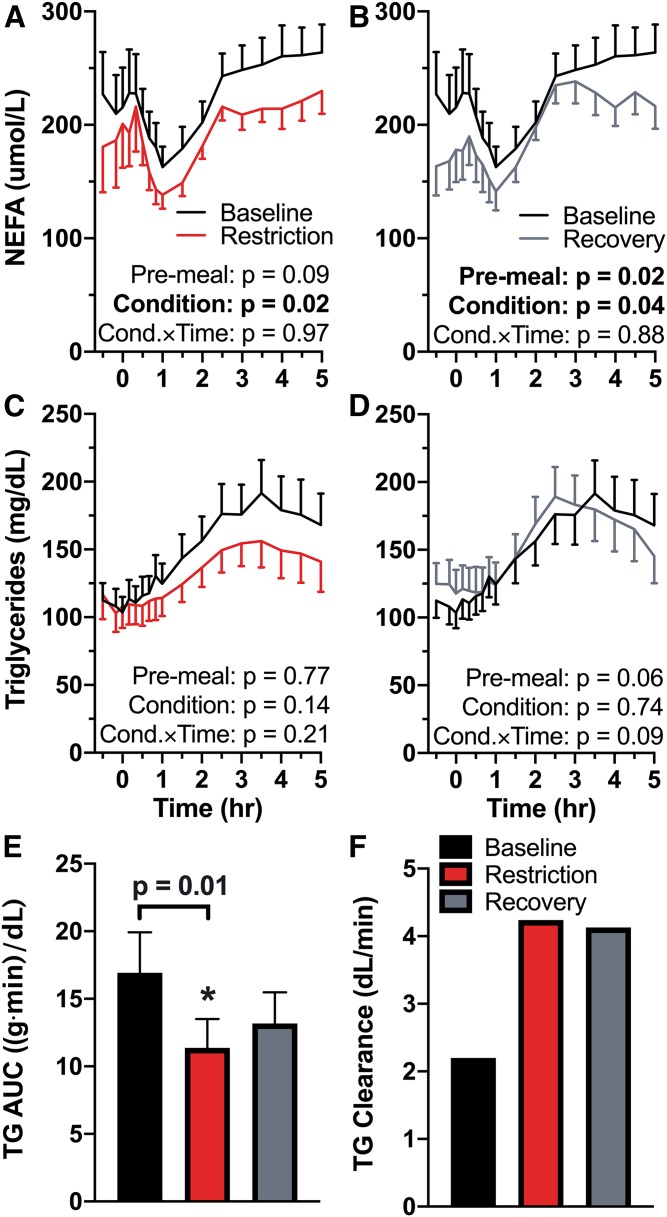
Pre-meal NEFAs were not significantly different during restriction compared with baseline (P = 0.09; Fig. 5A). However, they were significantly suppressed across the entirety of the HFD procedure (condition P = 0.02), with no difference in slope of change in response to the meal (condition × time P = 0.97). Pre-meal NEFAs were significantly suppressed after one night of recovery sleep compared with baseline (P = 0.02; Fig. 5B) and remained suppressed throughout the HFD procedure (condition P = 0.04). There was no interaction of condition × time in NEFAs during recovery compared with baseline (P = 0.88). In a post hoc analysis, postprandial TG AUC was not correlated with insulin AUC (P = 0.17).
Fig. 5.
Effects of sleep restriction on postprandial lipemia. A–E: Mean values are plotted ± SEM (n = 15). F: Population value (clearance was calculated using the mean). Significant P-values are in bold type. Line graphs are oriented relative to meal start time (first bite = time 0). A: Baseline versus restriction NEFAs during HFD. NEFAs were suppressed throughout the HFD during sleep restriction (P = 0.02). B: Baseline versus recovery HFD NEFAs. Pre-meal NEFAs were decreased in the recovery condition compared with baseline (P = 0.02). NEFAs remained suppressed throughout the HFD (P = 0.04). C: Baseline versus restriction HFD TGs. D: Baseline versus recovery HFD TGs. E: TG AUC during the HFD from minutes 0–300. Sleep restriction decreased TG AUC compared with baseline (P = 0.01). F: Population TG clearance calculated as dose per mean AUC. Cond.×Time, condition × time.
During sleep restriction, there was no difference in pre-meal TG compared with baseline (P = 0.77; Fig. 5C). There was neither an effect of condition (P = 0.14) nor a condition × time interaction (P = 0.21) in TG between baseline and restriction, but AUC of TG from minutes 0 to 300 was significantly decreased in sleep restriction compared with baseline (P = 0.01; Fig. 5E). Population TG clearance increased from 2.20 dl/min at baseline to 4.25 dl/min in the sleep restriction condition (Fig. 5F). Pre-meal TGs were not different in recovery compared with baseline (P = 0.06), there was no effect of condition (P = 0.74) or a condition × time interaction (P = 0.09; Fig. 5D). There was no significant difference in postprandial TG AUC between the baseline and recovery conditions (P = 0.13; Fig. 5E). Population TG clearance in recovery remained higher than baseline clearance at 4.13 dl/min (Fig. 5F).
DISCUSSION
This study examined whether the impaired glucose tolerance and altered lipid oxidation induced by four nights of 5 h TIB/night has real-world repercussions for metabolism by assessing the postprandial response to a standardized HFD in young healthy men. Furthermore, this study assessed whether one night of recovery sleep (10 h TIB opportunity) was sufficient to restore postprandial metabolic processes to baseline levels. This study extends from prior studies of meal digestion during sleep restriction by: 1) examining the effects of a high-fat mixed meal rather than a high-carbohydrate mixed meal; and 2) by administering the test meal in the evening, when many Americans eat their largest and most calorie-laden meal of the day. In healthy young men fed an HFD, four nights of 5 h TIB/night increased pre-meal and postprandial insulin without altering glucose levels, suppressed pre-meal and postprandial NEFAs, decreased TG AUC and increased TG clearance, decreased postprandial satiety without altering satiety hormones, and decreased the postprandial lipemia-induced increase in IL-6.
Sleep restriction increased pre-meal c-peptide and insulin levels. These elevations in preprandial insulin and c-peptide occurred despite no difference in pre-meal glucose, indicative of a pancreatic adjustment to decreased whole-body insulin sensitivity in order to maintain the glycemic homeostatic set point. Sleep restriction also increased postprandial insulin, but did not alter postprandial glucose or c-peptide responses. The increased postprandial plasma insulin values during sleep restriction, despite no difference in postprandial glucose levels or glucose AUC, further indicates metabolic compensation for decreased whole-body insulin sensitivity. Elevated postprandial insulin likely drove many of our lipemic findings, for example, the suppressed NEFA and increased TG clearance. Previous sleep restriction studies using test meals consumed in the morning found impaired glucose clearance without changes to insulin secretion, suggesting a decrement in pancreatic control of glycemia. However, with an evening meal administration, we found evidence of pancreatic compensation for impaired whole-body insulin sensitivity potentially due to a time-of-day (or circadian) effect on metabolism. One possible explanation for our findings could be the increased temporal distance from the nightly melatonin peak offset of an evening meal (65). Melatonin influences insulin secretion and glucose tolerance, and is altered by morning light exposure (even relatively dim light, such as in this study) (65–67). We did not measure melatonin, so this hypothesis requires further testing in future studies.
There was a significant consistent suppression of NEFAs in response to sleep restriction compared with baseline, and one night of recovery sleep was insufficient to restore NEFAs to baseline values (Fig. 5). NEFA release from adipose tissue is powerfully suppressed by insulin, so it is possible that the elevations in pre-meal and postprandial insulin during sleep restriction were at least partially responsible for the observed NEFA suppression. NEFAs remained suppressed in the recovery condition compared with baseline despite pre-meal and postprandial insulin levels returning to sleep-replete levels. These findings provide evidence that there may be additional physiological changes occurring in response to sleep restriction, such as changes to the rate of NEFA release from adipose tissue or utilization by peripheral tissues.
The postprandial lipemic response was significantly suppressed by sleep restriction. Compared with baseline, TG AUC was decreased by 32.8% and TG clearance was increased by 92.7%. In contrast to NEFAs, TG AUC was not significantly different from baseline following one night of 10 h TIB recovery sleep, even though TG clearance remained elevated. While it is possible that sleep restriction significantly disturbs the digestive and absorptive processes of the gut, which are under circadian control, available evidence suggests that increased TG clearance is more likely responsible for the observed differences (68, 69). Meal lipids are packaged into lipoproteins (chylomicrons and VLDLs) by the gut and liver for transport to peripheral tissues (17, 70, 71). Insulin stimulates LPL translocation to the endothelial surface, promoting uptake and clearance of postprandial lipoproteins from the blood (72–74). The elevated postprandial insulin during sleep restriction may increase LPL activity and thereby increase TG clearance and decrease postprandial TG AUC (72, 75). Although this physiology is well described, a post hoc analysis found no correlation between postprandial insulin and TG AUCs, indicating that there may be additional physiological mechanisms contributing to the observed changes in TG AUC and clearance. For example, prior sleep restriction studies have found an increased fasting ratio of peripheral lipid to carbohydrate oxidation (decreased fasting respiratory quotient) as well as evidence for increased hepatic fatty acid oxidation (increased β-hydroxy butyrate levels) (9, 22–24). Therefore, in addition to increased LPL activity, there may be a sleep restriction-induced shift in fuel selection favoring lipid clearance. The mechanisms underlying this shift in fuel selection preference and the tissue(s) in which this shift occurs remain unknown.
Compared with a baseline sleep-replete condition, we found no differences in pre-meal levels of the hunger and satiety hormones ghrelin, GLP-1, and leptin. Participants’ self-reported levels of hunger and satiety were not different prior to the meal start; after consuming the meal, however, participants were significantly less satiated during sleep restriction compared with baseline. One night of recovery sleep restored participant postprandial satiety ratings to baseline levels. There was a significant interaction of condition × time in the response of postprandial ghrelin during sleep restriction compared with baseline. We administered the hunger/satiety surveys to participants immediately following meal completion (no later than 20 min after meal start), so changes that occurred in ghrelin across the entirety of the HFD are unlikely to relate to the immediate postprandial decrease in fullness reported by participants in the restriction condition. It is possible that other satiety-signaling hormones that we did not measure in this study drove the decrease in participant-reported fullness.
IL-6 is a cytokine that is produced by immune cells, fibroblasts, and endothelial cells in many bodily tissues, including the adipose tissue, skeletal muscle, and liver (76). Visceral and omental adipose tissue produce approximately one-third of circulating plasma IL-6, and IL-6 levels correlate with visceral adiposity (77, 78). IL-6 signaling plays a role in both the initiation and resolution of inflammation and, although long-term elevation is associated with chronic disease, brief increases are a normal part of diurnal metabolism and meal digestion (76, 77). A number of studies have demonstrated an increase in inflammatory markers after a high-fat meal, but only IL-6 has such a robust response that it has consistently been demonstrated to increase postprandially across a large number of studies (79). In particular, IL-6 increases robustly following a high-fat or mixed meal, with little postprandial response to a carbohydrate meal (77). Evidence relating sleep restriction to IL-6 is mixed, although a recent meta-analysis found that increases in IL-6 are associated with sleep disturbances rather than restriction per se (80–82). We found the postprandial IL-6 response to be significantly blunted by sleep restriction. This was partially, but not fully, restored with one night of 10 h TIB recovery. Due to the exponential and non-normal nature of our IL-6 data, the data were transformed using a median split. This transformation explains the significance of the condition variable in the recovery IL-6 model despite the overlapping mean values. In post hoc analyses, nontransformed IL-6 was not correlated with either TG values or TG AUC; however, in an exploratory analysis, the interaction of time into procedure and TG values significantly predicted IL-6 values (P < 0.0001) in all conditions, supporting the hypothesis that postprandial IL-6 levels are a response to plasma levels of meal lipids. Although the possibility that sleep restriction acts independently on postprandial IL-6 evolution cannot be excluded, the most plausible explanation for the observed decrease in postprandial IL-6 is that the decreased TG AUC during sleep restriction induces a blunted postprandial IL-6 response.
In conclusion, this study examined the effects of sleep restriction on postprandial metabolism, particularly lipemia. We found that the postprandial TG lipemia following an evening meal is significantly suppressed by four nights of sleep restriction and restored with one night of recovery sleep. Decreased TG AUC during sleep restriction corresponded to an increased TG clearance, which remained elevated after one night of recovery sleep. Although they were not significantly correlated, elevated plasma insulin during sleep restriction likely drove the changes in postprandial TG. The postprandial increase in the inflammatory marker IL-6 mirrored the changes in TG with sleep restriction, also returning to baseline levels with one night of recovery sleep. Participants reported feeling less satiated following the meal during sleep restriction, despite no differences in hunger before or after the meal. Furthermore, we found that sleep restriction suppresses NEFAs both before and during an evening high-fat meal and that one night of recovery sleep is not sufficient to restore NEFAs to baseline values, despite recovery condition insulin returning to baseline levels. In the context of whole-body metabolism, this may indicate a shift in fuel selection, with preference given to lipids. It is possible that repeated or prolonged exposure to sleep restriction leads to counter-regulatory shifts in these mechanisms, explaining the association between short sleep and elevated TG in epidemiological studies. This study demonstrates that sleep restriction induces significant shifts in lipemia in response to a highly translational metabolic challenge, which, when taken together with epidemiological evidence, may be one factor contributing to the increased cardiometabolic risk associated with short sleep.
Limitations
This study is limited by relatively small sample size and limited population scope, as this pilot study only included young healthy men. Future studies should include women and older adults. Randomizing the baseline and restriction conditions to occur on separate laboratory stays with adequate recovery time in between could strengthen future studies. We did not assess gut motility, measure fat absorption efficiency, or directly measure TG clearance rates, so our study was unable to determine whether the observed decreases in postprandial lipemia were due to increased clearance of TGs from the plasma, or due to decreased absorption from the gut. Future studies utilizing labeled TG tracers, stool analyses of residual fat content, and quantitation of LPL activity could further identify the mechanistic shifts caused by sleep restriction.
Footnotes
Abbreviations:
- AUC
- area under the curve
- GLP-1
- glucagon-like peptide-1 (active)
- HFD
- high-fat dinner
- IL-6
- interleukin-6
- MCP-1
- monocyte chemoattractant protein-1
- PSG
- polysomnography
- TIB
- time in bed
This work was supported by a pilot grant (PI: A-M.C.) from the Pennsylvania State University Clinical and Translational Sciences Institute (funded by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant UL1TR002014) and institutional funds from the College of Health and Human Development of the Pennsylvania State University to A-M.C., G.C.S., and O.M.B. K.M.N. was supported, in part, by National Institutes of Health Grant T32GM108563 and the College of Health and Human Development of the Pennsylvania State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Pennsylvania State University. G.C.S. serves on the Scientific Advisory Board of Amarin Pharmaceuticals. Outside of the current work, O.M.B. received two subcontract grants to Pennsylvania State University from Mobile Sleep Technologies (NSF/STTR #1622766, NIH/NIA SBIR R43AG056250). The remaining authors have indicated no financial conflicts of interest relevant to the current study.
REFERENCES
- 1.Centers for Disease Control and Prevention 2017. Data and Statistics - Sleep and Sleep Disorders. Accessed February 14, 2019, at https://www.cdc.gov/sleep/data_statistics.html.
- 2.St-Onge M. P., Grandner M. A., Brown D., Conroy M. B., Jean-Louis G., Coons M., and Bhatt D. L.. 2016. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 134: e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandner M. A., Hale L., Moore M., and Patel N. P.. 2010. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med. Rev. 14: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks S., and Dinges D. F.. 2007. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 3: 519–528. [PMC free article] [PubMed] [Google Scholar]
- 5.Reutrakul S., and Van Cauter E.. 2018. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 84: 56–66. [DOI] [PubMed] [Google Scholar]
- 6.Rangaraj V. R., and Knutson K. L.. 2016. Association between sleep deficiency and cardiometabolic disease: implications for health disparities. Sleep Med. 18: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broussard J. L., Ehrmann D. A., Van Cauter E., Tasali E., and Brady M. J.. 2012. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann. Intern. Med. 157: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broussard J. L., Chapotot F., Abraham V., Day A., Delebecque F., Whitmore H. R., and Tasali E.. 2015. Sleep restriction increases free fatty acids in healthy men. Diabetologia. 58: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao M. N., Neylan T. C., Grunfeld C., Mulligan K., Schambelan M., and Schwarz J-M.. 2015. Subchronic sleep restriction causes tissue-specific insulin resistance. J. Clin. Endocrinol. Metab. 100: 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness K. M., Strayer S. M., Nahmod N. G., Chang A-M., Buxton O. M., and Shearer G. C.. 2019. Two nights of recovery sleep restores the dynamic lipemic response, but not the reduction of insulin sensitivity, induced by five nights of sleep restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316: R697–R703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen T. S., Jessen N., Jørgensen J. O. L., Møller N., and Lund S.. 2014. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 52: R199–R222. [DOI] [PubMed] [Google Scholar]
- 12.Holm C. 2003. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 13.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., and Sul H. S.. 2007. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27: 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maheux P., Azhar S., Kern P. A., Chen Y-D., and Reaven G. M.. 1997. Relationship between insulin-mediated glucose disposal and regulation of plasma and adipose tissue lipoprotein lipase. Diabetologia. 40: 850–858. [DOI] [PubMed] [Google Scholar]
- 15.Panarotto D., Remillard P., Bouffard L., and Maheux P.. 2002. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur. J. Clin. Invest. 32: 84–92. [DOI] [PubMed] [Google Scholar]
- 16.Wang H., and Eckel R. H.. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297: E271–E288. [DOI] [PubMed] [Google Scholar]
- 17.Feingold K. R., and Grunfeld C.. 2000. Introduction to Lipids and Lipoproteins. Updated edition, June 10, 2015. Accessed March 10, 2019, at https://www.ncbi.nlm.nih.gov/books/NBK305896.
- 18.Somerharju P., Kuusi T., Paltauf F., and Kinnunen P. K. J.. 1978. Stereospecificity of lipoprotein lipase is an intrinsic property of the active site of the enzyme protein. FEBS Lett. 96: 170–172. [DOI] [PubMed] [Google Scholar]
- 19.Muralidhar R. V., Chirumamilla R. R., Marchant R., Ramachandran V. N., Ward O. P., and Nigam P.. 2002. Understanding lipase stereoselectivity. World J. Microbiol. Biotechnol. 18: 81–97. [Google Scholar]
- 20.Kraemer F. B., and Shen W-J.. 2002. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 43: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 21.Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., and Lass A.. 2009. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50: 3–21. [DOI] [PubMed] [Google Scholar]
- 22.Hibi M., Kubota C., Mizuno T., Aritake S., Mitsui Y., Katashima M., and Uchida S.. 2017. Effect of shortened sleep on energy expenditure, core body temperature and appetite: a human randomised crossover trial. Sci. Rep. 7: 39640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shechter A., Rising R., Wolfe S., Albu J. B., and St-Onge M. P.. 2014. Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int. J. Obes. (Lond.). 38: 1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingenberg L., Chaput J-P., Holmbäck U., Jennum P., Astrup A., and Sjödin A.. 2012. Sleep restriction is not associated with a positive energy balance in adolescent boys. Am. J. Clin. Nutr. 96: 240–248. [DOI] [PubMed] [Google Scholar]
- 25.Chua E. C-P., Shui G., Cazenave-Gassiot A., Wenk M. R., and Gooley J. J.. 2015. Changes in plasma lipids during exposure to total sleep deprivation. Sleep. 38: 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aho V., Ollila H. M., Kronholm E., Bondia-Pons I., Soininen P., Kangas A. J., Hilvo M., Seppälä I., Kettunen J., Oikonen M., et al. . 2016. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci. Rep. 6: 24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vondra K., Brodan V., Dobiásová M., Vítek V., and Kopecká J.. 1986. Effect of sleep deprivation on cholesterol metabolism and triglyceridaemia in male volunteers. Eur. J. Appl. Physiol. Occup. Physiol. 55: 83–87. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds A. C., Dorrian J., Liu P. Y., Van Dongen H. P. A., Wittert G. A., Harmer L. J., and Banks S.. 2012. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 7: e41218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Keeffe M., Roberts A. L., Kelleman M., Roychoudhury A., and St-Onge M.P.. 2013. No effects of short-term sleep restriction, in a controlled feeding setting, on lipid profiles in normal-weight adults. J. Sleep Res. 22: 717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerkhofs M., Boudjeltia K. Z., Stenuit P., Brohée D., Cauchie P., and Vanhaeverbeek M.. 2007. Sleep restriction increases blood neutrophils, total cholesterol and low density lipoprotein cholesterol in postmenopausal women: A preliminary study. Maturitas. 56: 212–215. [DOI] [PubMed] [Google Scholar]
- 31.Boudjeltia K. Z., Faraut B., Stenuit P., Esposito M. J., Dyzma M., Brohée D., Ducobu J., Vanhaeverbeek M., and Kerkhofs M.. 2008. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: a pilot study. Vasc. Health Risk Manag. 4: 1467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedeltcheva A. V., Kessler L., Imperial J., and Penev P. D.. 2009. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J. Clin. Endocrinol. Metab. 94: 3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaput J-P., McNeil J., Després J-P., Bouchard C., and Tremblay A.. 2013. Short sleep duration as a risk factor for the development of the metabolic syndrome in adults. Prev. Med. 57: 872–877. [DOI] [PubMed] [Google Scholar]
- 34.Kaneita Y., Uchiyama M., Yoshiike N., and Ohida T.. 2008. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 31: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorvatn B., Sagen I. M., Øyane N., Waage S., Fetveit A., Pallesen S., and Ursin R.. 2007. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J. Sleep Res. 16: 66–76. [DOI] [PubMed] [Google Scholar]
- 36.Araghi M. H., Thomas G. N., and Taheri S.. 2012. The potential impact of sleep duration on lipid biomarkers of cardiovascular disease. Clin. Lipidol. 7: 443–453. [Google Scholar]
- 37.Knutson K. L., Spiegel K., Penev P., and Van Cauter E.. 2007. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 11: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buxton O. M., Cain S. W., O’Connor S. P., Porter J. H., Duffy J. F., Wang W., Czeisler C. A., and Shea S. A.. 2012. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4: 129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegel K., Leproult R., and Van Cauter E.. 1999. Impact of sleep debt on metabolic and endocrine function. Lancet. 354: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 40.Kolovou G. D., Mikhailidis D. P., Kovar J., Lairon D., Nordestgaard B. G., Ooi T. C., Perez-Martinez P., Bilianou H., Anagnostopoulou K., and Panotopoulos G.. 2011. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr. Vasc. Pharmacol. 9: 258–270. [DOI] [PubMed] [Google Scholar]
- 41.Pirillo A., Norata G. D., and Catapano A. L.. 2014. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 30: 1489–1503. [DOI] [PubMed] [Google Scholar]
- 42.Chan D. C., Pang J., Romic G., and Watts G. F.. 2013. Postprandial hypertriglyceridemia and cardiovascular disease: current and future therapies. Curr. Atheroscler. Rep. 15: 309. [DOI] [PubMed] [Google Scholar]
- 43.Ruddick-Collins L. C., Johnston J. D., Morgan P. J., and Johnstone A. M.. 2018. The Big Breakfast Study: chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 43: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antunes L. C., Levandovski R., Dantas G., Caumo W., and Hidalgo M. P.. 2010. Obesity and shift work: chronobiological aspects. Nutr. Res. Rev. 23: 155–168. [DOI] [PubMed] [Google Scholar]
- 45.Garaulet M., Gómez-Abellán P., Alburquerque-Béjar J. J., Lee Y-C., Ordovás J. M., and Scheer F. A. J. L.. 2013. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. (Lond.). 37: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakubowicz D., Barnea M., Wainstein J., and Froy O.. 2013. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 21: 2504–2512. [DOI] [PubMed] [Google Scholar]
- 47.Knutsson A., Karlsson B., Örnkloo K., Landström U., Lennernäs M., and Eriksson K.. 2002. Postprandial responses of glucose, insulin and triglycerides: influence of the timing of meal intake during night work. Nutr. Health. 16: 133–141. [DOI] [PubMed] [Google Scholar]
- 48.Aparecida Crispim C., and Carliana Mota M.. 2019. New perspectives on chrononutrition. Biol. Rhythm Res. 50: 63–77. [Google Scholar]
- 49.St-Onge M. P., Ard J., Baskin M. L., Chiuve S. E., Johnson H. M., Kris-Etherton P., and Varady K.. 2017. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 135: e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y. 2003. Association between eating patterns and obesity in a free-living US adult population. Am. J. Epidemiol. 158: 85–92. [DOI] [PubMed] [Google Scholar]
- 51.Cleator J., Abbott J., Judd P., Sutton C., and Wilding J. P. H.. 2012. Night eating syndrome: implications for severe obesity. Nutr. Diabetes. 2: e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fong M., Caterson I. D., and Madigan C. D.. 2017. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta-analysis. Br. J. Nutr. 118: 616–628. [DOI] [PubMed] [Google Scholar]
- 53.McHill A. W., Phillips A. J., Czeisler C. A., Keating L., Yee K., Barger L. K., Garaulet M., Scheer F. A., and Klerman E. B.. 2017. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 106: 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garaulet M., and Gómez-Abellán P.. 2014. Timing of food intake and obesity: A novel association. Physiol. Behav. 134: 44–50. [DOI] [PubMed] [Google Scholar]
- 55.Lee R. A., Harris C. A., and Wang J-C.. 2018. Glucocorticoid receptor and adipocyte biology. Nucl. Receptor Res. 5: 101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J-C., Gray N. E., Kuo T., and Harris C. A.. 2012. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattson M. P., Allison D. B., Fontana L., Harvie M., Longo V. D., Malaisse W. J., Mosley M., Notterpek L., Ravussin E., Scheer F. A. J. L., et al. . 2014. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA. 111: 16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wittmann M., Dinich J., Merrow M., and Roenneberg T.. 2006. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 23: 497–509. [DOI] [PubMed] [Google Scholar]
- 59.Frankenfield D., Roth-Yousey L., and Compher C.. 2005. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J. Am. Diet. Assoc. 105: 775–789. [DOI] [PubMed] [Google Scholar]
- 60.Harris J. A., and Benedict F. G.. 1918. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA. 4: 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marino M., Li Y., Rueschman M. N., Winkelman J. W., Ellenbogen J. M., Solet J. M., Dulin H., Berkman L. F., and Buxton O. M.. 2013. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 36: 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berry R. B., Brooks R., Gamaldo C., Harding S. M., Lloyd R. M., Quan S. F., Troester M. T., and Vaughn B. V.. 2017. AASM scoring manual updates for 2017 (version 2.4). J. Clin. Sleep Med. 13: 665–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berry R. B., Brooks R., Gamaldo C. E., Harding S. M., Lloyd R. M., Marcus C. L., and Vaughn B. V.. 2015. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Accessed February 5, 2019, at https://aasm.org/resources/pdf/scoring-manual-preface.pdf. [Google Scholar]
- 64.Parker B. A., Sturm K., MacIntosh C. G., Feinle C., Horowitz M., and Chapman I. M.. 2004. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 58: 212–218. [DOI] [PubMed] [Google Scholar]
- 65.Eckel R. H., Depner C. M., Perreault L., Markwald R. R., Smith M. R., McHill A. W., Higgins J., Melanson E. L., and Wright K. P.. 2015. Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr. Biol. 25: 3004–3010. [DOI] [PubMed] [Google Scholar]
- 66.McMullan C. J., Curhan G. C., Schernhammer E. S., and Forman J. P.. 2013. Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am. J. Epidemiol. 178: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma S., Singh H., Ahmad N., Mishra P., and Tiwari A.. 2015. The role of melatonin in diabetes: therapeutic implications. Arch. Endocrinol. Metab. 59: 391–399. [DOI] [PubMed] [Google Scholar]
- 68.Hussain M. M., and Pan X.. 2015. Circadian regulators of intestinal lipid absorption. J. Lipid Res. 56: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iqbal J., and Hussain M. M.. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karpe F., Steiner G., Olivecrona T., Carlson L. A., and Hamsten A.. 1993. Metabolism of triglyceride-rich lipoproteins during alimentary lipemia. J. Clin. Invest. 91: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hussain M. M. 2014. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 25: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braun J. E., and Severson D. L.. 1992. Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem. J. 287: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karpe F., Bickerton A. S., Hodson L., Fielding B. A., Tan G. D., and Frayn K. N.. 2007. Removal of triacylglycerols from chylomicrons and VLDL by capillary beds: the basis of lipoprotein remnant formation. Biochem. Soc. Trans. 35: 472–476. [DOI] [PubMed] [Google Scholar]
- 74.Bickerton A. S. T., Roberts R., Fielding B. A., Hodson L., Blaak E. E., Wagenmakers A. J. M., Gilbert M., Karpe F., and Frayn K. N.. 2007. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 56: 168–176. [DOI] [PubMed] [Google Scholar]
- 75.Lewis G. F., Uffelman K. D., Szeto L. W., and Steiner G.. 1993. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 42: 833–842. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt-Arras D., and Rose-John S.. 2016. IL-6 pathway in the liver: from physiopathology to therapy. J. Hepatol. 64: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 77.Maggio M., Guralnik J. M., Longo D. L., and Ferrucci L.. 2006. Interleukin-6 in aging and chronic disease: a magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 61: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fried S. K., Bunkin D. A., and Greenberg A. S.. 1998. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83: 847–850. [DOI] [PubMed] [Google Scholar]
- 79.Emerson S. R., Kurti S. P., Harms C. A., Haub M. D., Melgarejo T., Logan C., and Rosenkranz S. K.. 2017. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv. Nutr. 8: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Redwine L., Hauger R. L., Gillin J. C., and Irwin M.. 2000. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 85: 3597–3603. [DOI] [PubMed] [Google Scholar]
- 81.Irwin M. R., Olmstead R., and Carroll J. E.. 2016. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry. 80: 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mullington J. M., Simpson N. S., Meier-Ewert H. K., and Haack M.. 2010. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 24: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]