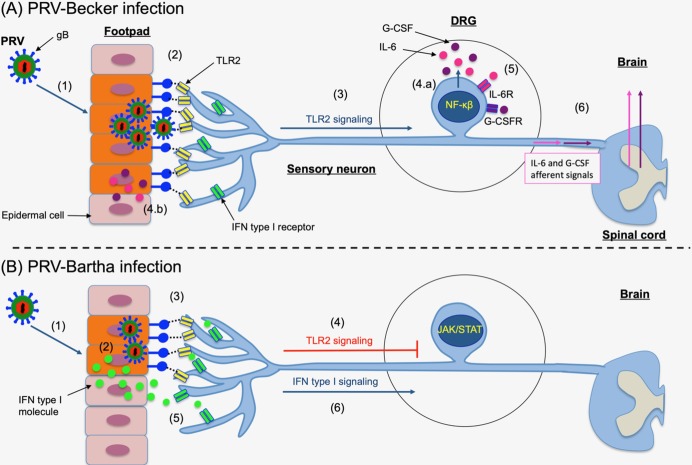
Fig 7. Model of PRV infection and priming of DRG neurons to an inflammatory state versus antiviral state at very early time post-infection.
(A) (1) PRV-Becker efficiently replicates and spreads in the epidermal cells of the mouse footpad. (2) PRV gB expressed on infected epidermal cells or on new progeny virions may interact with TLR2 expressed on axonal terminals of DRG neurons that are innervating the footpad. (3) This high level gB-TLR2 interaction activates TLR2 signaling pathway through NF-κβ in DRG neurons and leads to the synthesis of G-CSF and IL-6 molecules. (4) Both cytokines are released in (a) DRG neurons and (b) locally at axon terminals innervating the footpad. (5) G-CSF and IL-6 molecules can directly interact with their receptors (G-CSFR and IL6R) expressed on DRG neurons priming them to an inflammatory state with global effects. (6) G-CSF- and IL-6-mediated signaling pathways may increase the excitability of DRG nociceptors and facilitate synaptic transmission of specific IL-6 and G-CSF afferent signals to the CNS. (B) (1) PRV-Bartha does not replicate and spread efficiently in epidermal cells. (2) The infection induces a rapid type I IFN response in cells. (3) Only a few infected epidermal cells and progeny virions expressing gB on their surfaces can interact with TLR2 found on DRG peripheral axons. (4) Low level gB-TLR2 engagement is below the threshold for activation of TLR2-mediated cytokine signaling pathway. (5) In contrast, type I IFN molecules released from infected epidermal cells bind to their receptors and directly prime DRG neurons to an antiviral state, through activation of type I IFN signaling pathway (JAK/STAT) (6).