Table 1.
Synthesis of enantioenriched cyclobutanones.
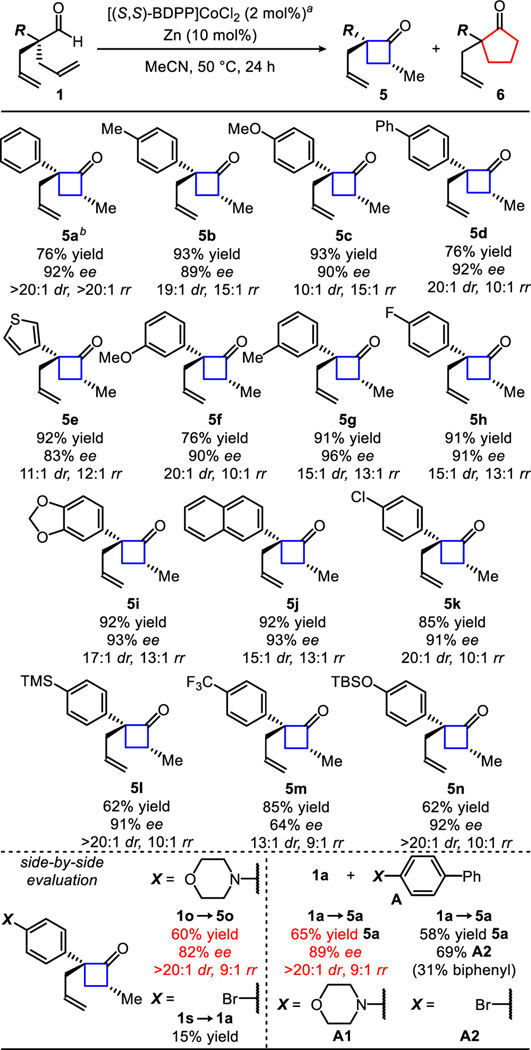 |
Reaction conditions: 1 (0.1 mmol), [(S,S)-BDPP]CoCl2 (2 mol%), zinc (10 mol%), MeCN (0.5 mL), 50 °C, 24 h.
Gram-scale reaction (1a 6.40 mmol; [(S,S)-BDPP]CoCl2 (0.128 mmol, 2 mol%), zinc (0.640 mmol, 10 mol%), MeCN (32 mL, 0.2M), 50 °C, 24 h).
Robustness evaluation: Identical conditions above (a) + A [additive] (0.1 mmol, 1 equiv.).
Isolated yields of 5. Diastereo- and regioisomeric ratio determined by GC-FID analysis of the unpurified reaction mixture. Enantioselectivies determined by SFC analysis of the purified ketone.
