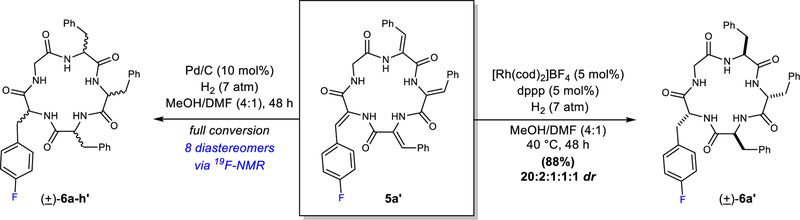
Figure 3. Examining the hydrogenation of cyclic dehydropeptide 5a’ gives mechanistic insight.
Pictured on the left is the hydrogenation of cyclic dehydropeptide 5a’ using heterogeneous catalysis. Shown on the right is the hydrogenation of cyclic dehydropeptide 5a’ using achiral rhodium catalysis. cod, 1,5-cyclooctadiene; dppp, 1,3-bis(diphenylphosphino)propane; atm, atmosphere. The inclusion of a prime in the compound number indicates it is the fluorinated analog. E.g. 5a = cyclo(Gly-ΔPhe-ΔPhe-ΔPhe-ΔPhe) 5a’ = cyclo(Gly-ΔPhe-ΔPhe-ΔPhe-ΔPhe(4-F)), ΔPhe, dehydrophenylalanine.