Figure 2. Some approaches in the minimalist design of membrane sequences.
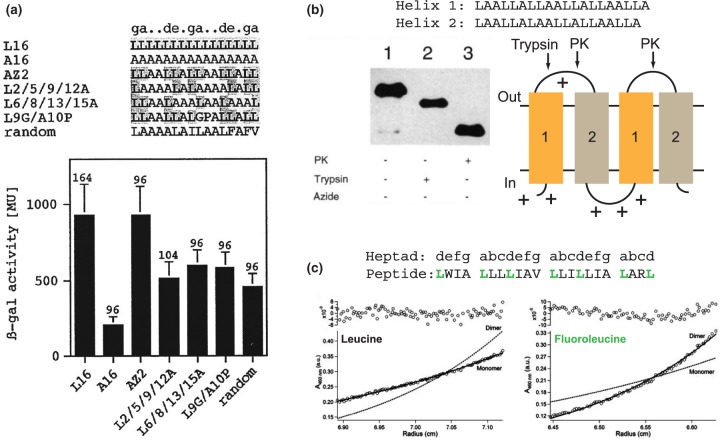
(a) A transcriptional activation assay shows that polyleucine sequences self-assemble in vivo. Mutational analysis confirms the importance of leucine at heptad positions a and d, suggesting a leucine zipper interface. Republished with permission of The American Society for Biochemistry and Molecular Biology from Gurezka et al. [44] © 1999. Permission conveyed through Copyright Clearance Center, Inc. (b) Topological control of a recombinant minimal membrane protein. Two slightly different sequences for minimal TM helices are used to assemble a four-helix construct as shown, and charged residues are introduced at the N-terminus and intracellular loop to control topology according to the ‘positive inside’ rule. Topology within the E. coli inner membrane is assessed by proteolysis of the exposed periplasmic loops, which leads to the predictable differences in gel migration shown. PK, Proteinase K. Left panel adapted by permission from Springer Nature. Whitley et al. [81] © 1994. (c) Introducing hexafluoroleucine at heptad positions a and d encourages dimerisation of a minimal peptide as determined by equilibrium analytical ultracentrifgation. Adapted with permission from Bilgiçer and Kumar [86]. © 2004 National Academy of Sciences, U.S.A.