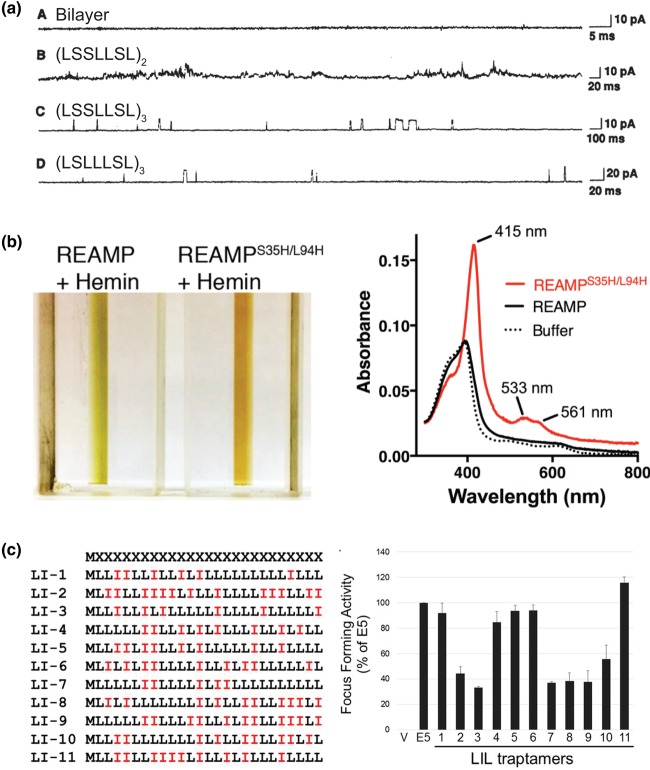
Figure 3. Examples of functionality in minimal membrane proteins.
(a) Electrophysiology of synthetic lipid bilayers containing minimal ion channels. Panel A shows a negative control without any peptide; Panel B is control using a short peptide that cannot span the bilayer; Panel C is peptide with sequence (LSSLLSL)3; and Panel D is (LSLLLSL)3. From Lear et al. [80]. Reprinted with permission from AAAS, adapted with permission of the authors. (b) A minimal 4-helix bundle (‘REAMP'), in which each helix has the sequence LLLLSGLGLLLLSLLGLLLLS, can be induced to bind a haem cofactor via a bis-Histidine site. The reddish-brown colour and the spectroscopic fingerprint of the oxidised hemoprotein are both reminiscent of natural cytochromes. Adapted from ref. [82] https://creativecommons.org/licenses/by/4.0/. (c) A series of minimal single TM helices — termed ‘LIL proteins’ — interact with the PDGF-β receptor and transform mammalian cells. The activity of each sequence LI1–11 is compared with a natural activator, the small oncoprotein E5. Adapted with permission from Heim et al. [99].