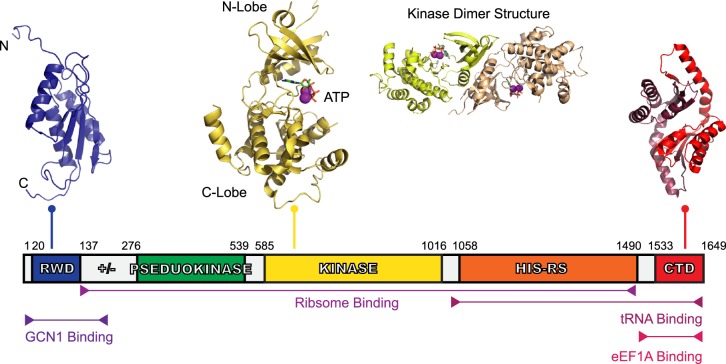
Figure 1. Domain organisation of human GCN2.
GCN2 is a 1649 amino acid protein with five conserved domains: the RWD, the pseudokinase domain, kinase domain, HisRS-Like domain, and CTD. Linking the RWD and pseudokinase domain is ‘charged linker’ (labelled ‘+/−’) region also. The structure of GCN2's RWD domain from mouse was determined using nuclear magnetic resonance (NMR) spectroscopy [39], showing an α + β sandwich fold (PDB ID: 1UKX). The charged linker, a stretch of arginine, lysine, glutamate, and aspartate residues, precedes the pseudokinase domain — the structure of which has yet to be determined — and was identified as a pseudokinase domain due to the sequence similarity to typical kinase domains, however, it lacks key catalytic residues. The kinase domain, shows a typical kinase domain structure (PDB ID: 1ZYD) [10], and is presented as both a monomer and dimer with its associated ATP and Mg2+. Following the kinase domain is the HisRS-like domain, a domain essential for GCN2 activity in vivo [14] which interacts directly with tRNA [15]. Finally, there is the CTD, shown here using the dimeric mouse CTD structure (PDB ID: 4OTN) [27]. The CTD is constitutively dimeric, similar to the kinase domain. There is a species-dependent interaction of the CTD with ribosomes also, which is reliant on three lysine residues found in yeast. Yeast CTDs co-migrate with ribosomes on a sucrose gradient, but this is not the case with the mouse CTD where these lysine residues are absent.