Fig. 3. Mechanism of the reversibly switching interfacial properties.
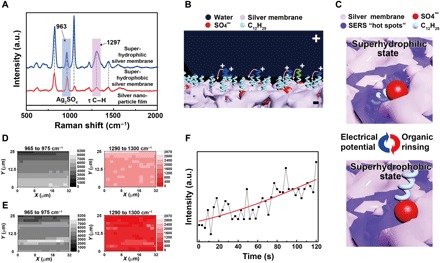
(A) SERS spectra of the silver porous membrane at superhydrophilic state and superhydrophobic state and the electrodeposited silver nanoparticle film in pure silver nitrate aqueous solutions. The SERS peaks located at 963 and 1297 cm−1 are assigned to silver sulfate and the torsional vibration mode (τ) of C─H in the dodecyl chains, respectively. a.u., arbitrary units. (B) Schematic illustration of the orientation change of the dodecyl chains under an electrical potential. Positive charges will accumulate at the tips of the dodecyl chains contacting water, rotating them toward the negatively charged silver porous membrane. (C) Schematic illustration of the SERS intensity evolution of the dodecyl chains at different wetting states. At hydrophilic state, dodecyl chains are exposed to the SERS “hot spots” existing within the pores of the silver membrane, resulting in strong SERS signals. At hydrophobic state, the dodecyl chains are far away from the SERS hot spots, demonstrating weak SERS signals. (D and E) The SERS mapping results of silver sulfate and the dodecyl chains when the porous membrane is superhydrophilic and superhydrophobic, respectively. (F) The intensity evolution of the 1297-cm−1 SERS peak from dodecyl chains at a specific location as the electrical potential was applied to the superhydrophobic silver porous membrane (photo credit: Yue Liu, Zhejiang University).
