Figure 2.
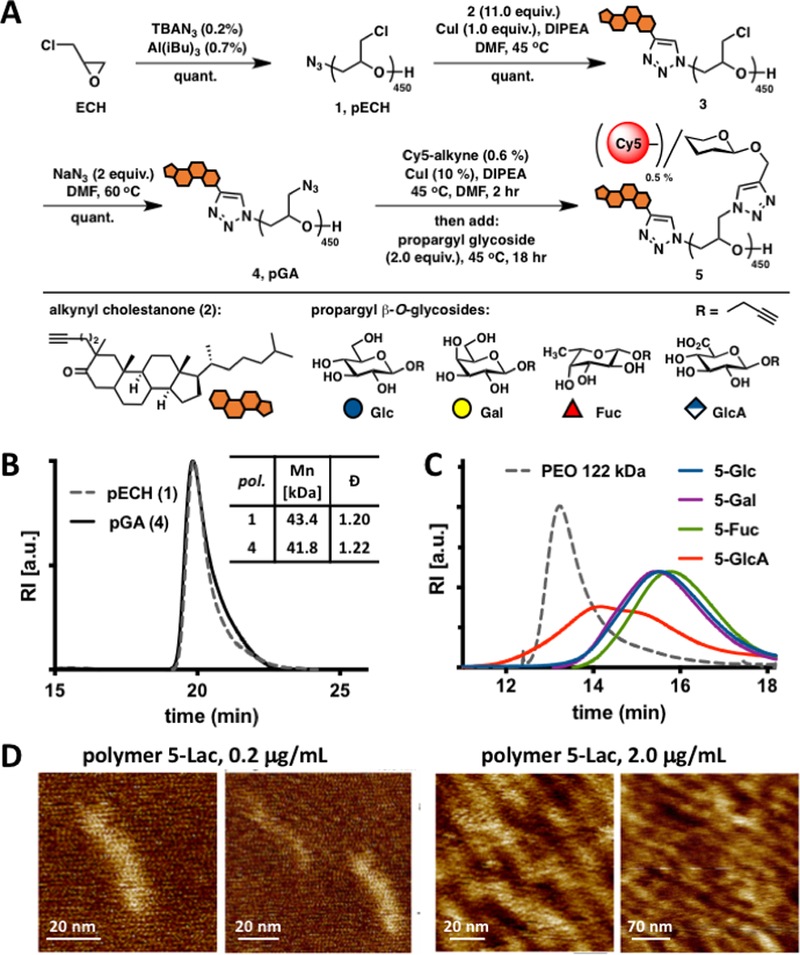
Synthesis and characterization of mucin mimetics 5. (A) Glycopolymers 5 were generated from poly(epichlorohydrin) 1 via chloride-to-azide sidechain substitution followed by the CuAAC conjugation of propargyl β-O-glycosides (Glc, Gal, Fuc, and GlcA). The polymers were furnished with a hydrophobic cholestanone moiety for anchoring into cell membranes and a Cy5 fluorescent tag for imaging and quantification. (B) SEC analysis indicated narrow molecular weight and chain-length distributions before and after the chloride-to-azide exchange. (C) Glycopolymers 5 exhibited increased retention in aqueous SEC compared to a PEO standard of similar molecular weight (122 kDa), a characteristic behavior of glycopolymers with extended molecular conformations. (D) AFM imaging of lactose-modified glycopolymers confirmed elongated, mucin-like morphology of the PEG-based glycopolymers.
