Figure 4.
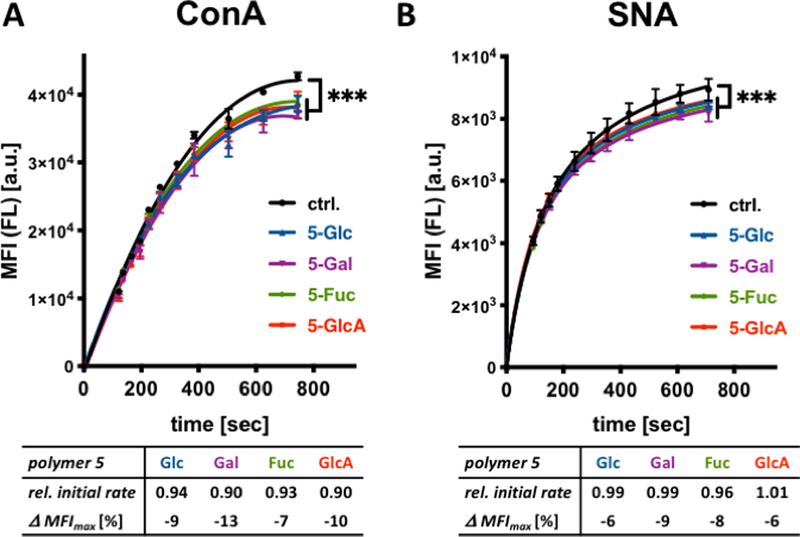
Association of ConA and SNA lectins with glycocalyx-remodeled RBCs. (A) Binding of fluorescein (FL)-labeled lectins to remodeled cells were assessed via flow cytometry. The presence of spectator glycopolymers 5 at the surface of RBCs attenuates both the initial rate as well as saturation binding of ConA to cell surface glycans. (B) Glycopolymers 5 have no effect on the initial rates of SNA binding but inhibit lectin association near saturation. Relative initial rates were calculated from the linear regions of lectin binding curves and normalized to control cells without polymer treatment. Δ MFImax corresponds to the change in median fluorescence intensity of cells at saturation lectin binding. (ANOVA, Tukey’s multiple comparisons test; p*** < 0.001).
