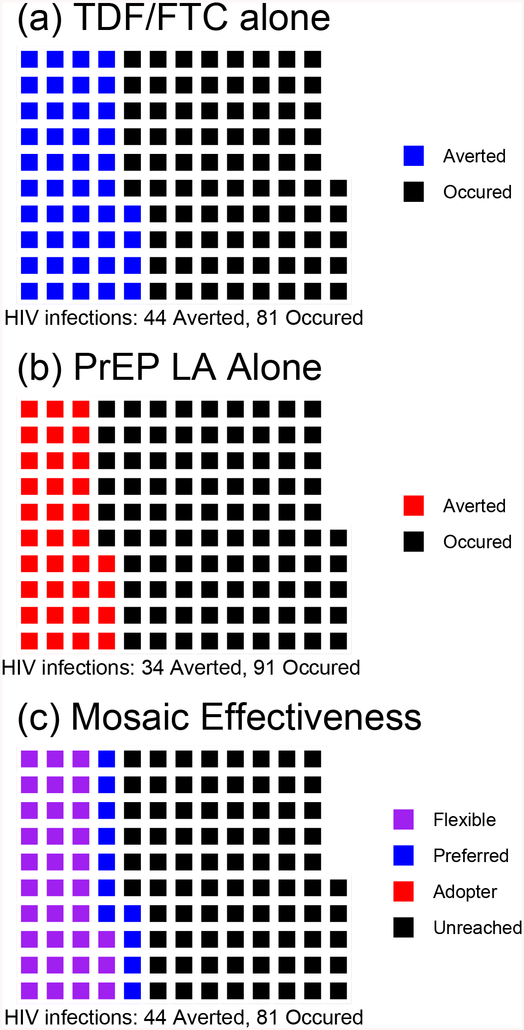
Figure 2. Population+ of 125 Who Would Seroconvert without PrEP. (a) TDF/FTC* Alone (b) PrEP LA** Alone (c) TDF/FTC and PrEP LA Offered as Choice: Redundant Scenario.
Note:
Panel 2 (a) Blue: Infections averted by TDF/FTC
Panel 2 (b) Red: Infections averted by PrEP LA
Latent Strata in Panel 2 (c)
Purple: Flexible (infections prevented by use of either TDF/FTC or PrEP LA), n=33
Blue: Pill-Preferred (infections averted by TDF/FTC but not by the PrEP LA), n=11
Red: PrEP LA Adopters (infected if offered TDF/FTC but averted by the PrEP LA), n=0
Black: Unreached (infected if provided TDF/FTC or PrEP LA), n=81
+ In this Figure, the same 125 individuals are displayed under three scenarios. Figures in the same position across the graphs identify the same individual.
*TDF/FTC: co-formulated tenofovir disoproxil fumarate and emtricitabine
**PrEP LA: non-daily and/or non-oral pre-exposure prophylaxis agent