Figure 1.
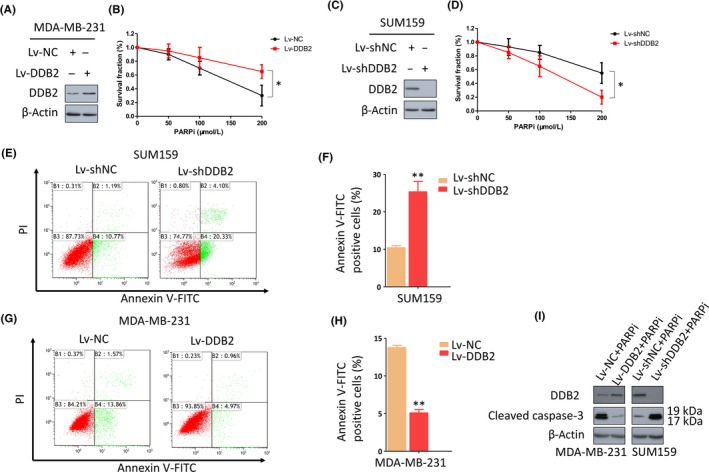
DNA damage binding protein 2 (DDB2) depletion confers sensitivity to poly ADP‐ribose polymerase inhibitors (PARPi) in triple‐negative breast cancer cells. A, MDA‐MB‐231 cells that expressed low levels of DDB2 were transfected with either a control lentivirus (Lv‐NC) or a DDB2 lentivirus (Lv‐DDB2). Immunoblotting was undertaken to evaluate the upregulation efficiency of DDB2 by Lv‐DDB2. β‐Actin was detected as a loading control. B, Clonogenic formation assay to evaluate the sensitivity of these cells to a PARPi. *P < .05. C, SUM159 cells that expressed high levels of DDB2 were transfected with either a control shRNA (Lv‐shNC) or DDB2 shRNA (Lv‐shDDB2). Immunoblotting was used to evaluate the knockdown efficiency of shDDB2. β‐Actin was detected as a loading control. D, Clonogenic formation assay to evaluate the sensitivity of these cells to a PARPi. *P < .05. E,F, DDB2‐depleted SUM159 cells were exposed to 150 μmol/L PARPi for 24 h, and apoptosis was measured by flow cytometry. Data are shown as the mean of 3 independent experiments ± SEM. **P < .01. G‐I, DDB2‐overexpressed MDA‐MB‐231 cells were exposed to 150 μmol/L PARPi for 24 h, and apoptosis was measured by flow cytometry. Data are shown as the mean of 3 independent experiments ± SEM. **P < .01. I, Western blot analysis of cleaved caspase‐3 expression in DDB2‐depleted SUM159 cells or DDB2‐overexpressing MDA‐MB‐231 cells in the presence of PARPi. β‐Actin served as the loading control
