Figure 4.
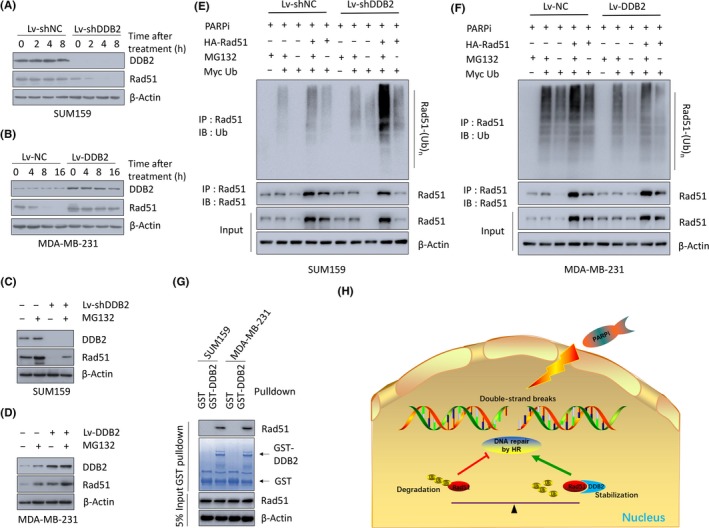
DNA damage binding protein 2 (DDB2) depletion increases Rad51 polyubiquitination and proteasomal degradation, leading to defective homologous recombination (HR) repair and sensitivity to poly ADP‐ribose polymerase inhibitors (PARPi). A, SUM159 DDB2‐depleted or control cells were pretreated for 15 min with 20 mmol/L cycloheximide followed by 150 μmol/L PARPi. DDB2 depletion decreased the apparent half‐life of the Rad51 protein in the presence of cycloheximide. B, MDA‐MB‐231 DDB2‐overexpression or control cells were pretreated for 15 min with 20 mmol/L cycloheximide followed by 150 μmol/L PARPi. DDB2 overexpression increased the apparent half‐life of the Rad51 protein in the presence of cycloheximide. C, Western blot analysis of DDB2, Rad51, and β‐actin in SUM159 cells transduced with DDB2 shRNA (Lv‐shDDB2) or control shRNA after treatment with 150 μmol/L PARPi in the absence or presence of 10 mmol/L MG132. D, Western blot analysis of DDB2, Rad51, and β‐actin in MDA‐MB‐231 cells transduced with DDB2 RNA (Lv‐DDB2) or control RNA after treatment with 150 μmol/L PARPi in the absence or presence of 10 mmol/L MG132. E, Level of Rad51 ubiquitination was detected in SUM159 DDB2‐depleted or control cells 2 h after PARPi removal. F, Level of Rad51 ubiquitination was detected in MDA‐MB‐231 DDB2‐overexpressing or control cells 2 h after PARPi removal. G, Cell lysates of SUM159 and MDA‐MB‐231 cells were incubated with bead‐bound GST or GST‐DDB2. Proteins retained on Sepharose were then subjected to immunoblotting with anti‐Rad51 Abs. H, Schematic model of the function of DDB2 on Rad51, HR repair, and the sensitivity to PARPi. IB, immunoblotting agent; IP, immunoprecipitant
