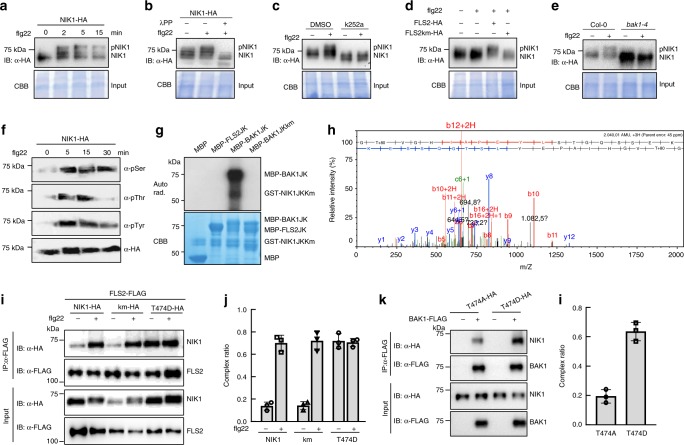
Fig. 5.
Flg22 induces BAK1-mediated NIK1 phosphorylation, which enhances NIK1’s affinity for receptors. a Flg22 perception triggers NIK1 rapid mobility shift. Protoplasts were transfected with NIK1-HA and treated with 100 nM flg22 for the indicated time points. Total input proteins were stained with Coomassie brilliant blue staining (CBB). b Verification of NIK1 in vivo phosphorylation by λPP treatment. Protein extracts from protoplasts transfected with NIK1-HA were treated with λPP following the standard protocol. c The kinase inhibitor K252a blocks flg22-induced NIK1 mobility shift. K252a was applied 1 h before flg22 treatment. Controls were solvent (DMSO) treatment. d Flg22-induced NIK1 phosphorylation requires FLS2 and its kinase activity. Protoplasts isolated from fls2 mutants were transfected with NIK1-HA and empty vector control, FLAG-tagged FLS2 or FLS2 kinase mutant (FLS2km). e Flg22-mediated NIK1 phosphorylation requires BAK1. NIK1-HA was expressed in protoplasts of Col-0 or bak1-4 mutants and flg22 was applied 10 min before samples collection. f Flg22-induced in vivo phosphorylation of NIK1 detected by different phospho-antibodies. Seedlings of NIK1-HA-overexpressing lines were treated with 100 nM flg22 for the indicated time points. NIK1-HA was immunoprecipitated from total protein extracts, fractionated by SDS-PAGE and immunoblotted with α-phosphoserine (α-pSer), α-phosphothreonine (α-Thr), α-phosphotyrosine (α-Tyr) and α-HA antibodies. g BAK1, but not FLS2, directly phosphorylates the NIK1 cytosolic domain. An in vitro kinase assay was performed using MBP-FLS2JK or MBP-BAK1JK as a kinase and GST-NIK1JKKm as the substrate. Phosphorylation was analysed by autoradiography (Upper), and the protein loading was shown by CBB (Lower). h NIK1 Thr474 is phosphorylated by BAK1 in vitro as shown by MS analysis. i Phosphorylation of NIK1 promotes its interaction with FLS2. FLS2-FLAG was co-expressed with NIK1-HA, NIK1Km-HA or NIK1-T474D-HA in protoplasts. Co-IP was performed with α-FLAG Agarose (IP: α-FLAG), and the proteins were immunoblotted with an α-HA antibody (IB: α-HA). j Quantitative data (n = 30 for h. k A NIK1 phosphomimetic form shows stronger interaction with BAK1. Co-IP assay was performed with the sample co-expressing BAK1-FLAG and T474A-HA or T474D-HA using α-FLAG Agarose. l Quantitative data from three biological replicates for j. Source data are provided as a Source Data file