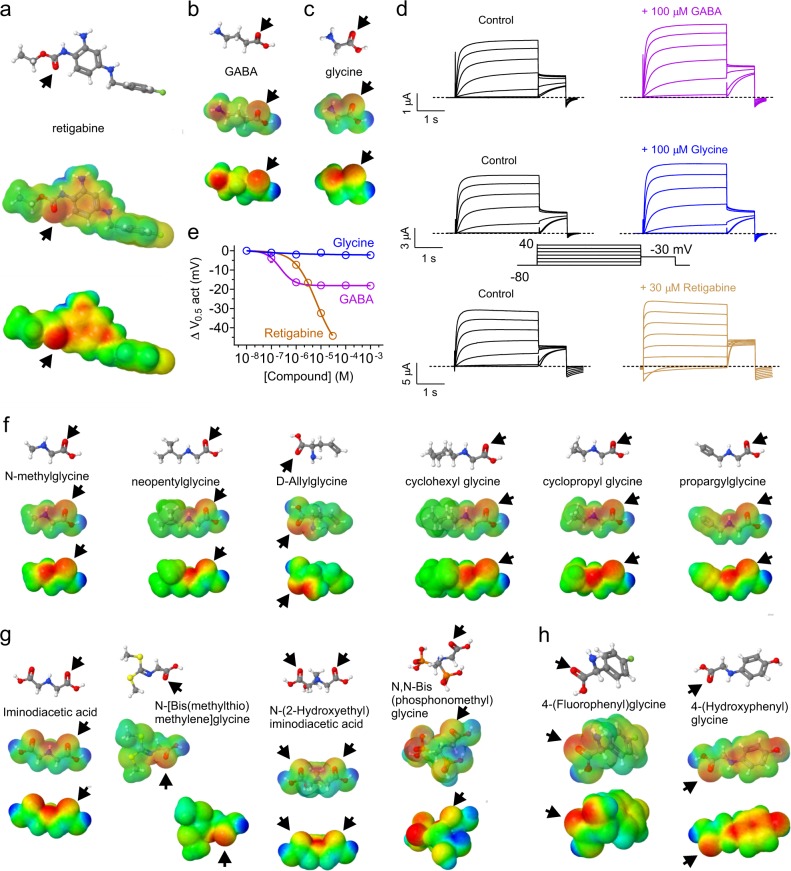
Fig. 1.
In silico engineering predicted KCNQ-opening properties into glycine. a Retigabine structure, electrostatic surface potentials (red, electron-dense; blue, electron-poor; green, neutral) and an overlay of the two, all calculated and plotted using Jmol. Arrow carbonyl oxygen. b GABA, parameters as in (a). c Glycine, parameters as in (a). d Mean traces showing effects of GABA, glycine and retigabine on KCNQ2/3 channels expressed in Xenopus oocytes (n = 4–6). Voltage protocol (inset) was used for all TEVC recordings in this study unless otherwise indicated. e KCNQ2/3 dose response to glycine, GABA and retigabine, quantified from recordings as in (d) as the shift in voltage dependence of activation (ΔV0.5act) measured from the tail current. Error bars indicate SEM; n = 4–6. f Structures and surface potential plots (as in (a)) for the simple glycine derivatives indicated; arrows, carbonyl oxygen. g Structures and surface potential plots (as in a) for the double-carbonyl or branched glycine derivatives indicated; arrows, carbonyl oxygen. h Structures and surface potential plots (as in (a)) for the glycine derivatives bearing a phenyl ring; arrows indicate carbonyl group