Abstract
This study aimed to assess the prevalence and related factors of obesity-related hypertension among adults aged 40 to 79 years in Southwest China. From September 2013 to March 2014, a multi-stage, stratified sampling method was conducted on 10,589 people aged 40 to 79 years and living in Chengdu and Chongqing investigated by using a questionnaire and performing physical and biochemical measurements. The prevalence of obesity-related hypertension and hypertension overall (systolic ≥130 mmHg and/or diastolic ≥80 mmHg or treated hypertension) was 22.8% and 57.4%, respectively, among all participants. For obesity-related hypertension, the prevalence was higher in women than in men (24.7% versus 19.4%, p < 0.001). For people in the age ranges of 40–49, 50–59, 60–69, and ≥70, the prevalence of obesity-related hypertension were 11.8%, 22.6%, 30.7%, and 36.6%, respectively. Participants with obesity-related hypertension as opposed to those with non-obesity-related hypertension had a higher prevalence of hypertriglyceridemia, high low-density lipoprotein cholesterolemia, diabetes, and hyperuricemia (all p < 0.05). Multivariate logistic regression analysis showed that age, female gender, current smoking, hypertriglyceridemia, diabetes and family history of hypertension were all positively correlated with obesity-related hypertension, whereas higher education level and having spouse were negatively correlated with obesity-related hypertension. The prevalence of obesity-related hypertension was high among adults aged 40 to 79 years in Southwest China. Cardiometabolic abnormalities among participants with obesity-related hypertension were more serious and frequently present than in those with non-obesity-related hypertension. Aggressive and holistic strategies aiming at the prevention and treatment of obesity-related hypertension are needed.
Subject terms: Cardiology, Hypertension
Introduction
Hypertension is a major risk factor that leads to myocardial infarction, stroke, renal failure, and death1,2. Obesity raises the risk of morbidity from hypertension, dyslipidemia, diabetes, coronary heart disease (CHD), stroke and heart failure3–6. Hypertension and obesity are both associated with increased risks of all-cause and cardiovascular mortality7–9, and they often occur together10. Socioeconomic and demographic transitions occurring in many developing countries have contributed to the burden of hypertension and obesity2,11, and the transition of morbidity from communicable diseases to non-communicable diseases12,13. With the rapid industrialization and urbanization seen in recent decades, the prevalence of hypertension and obesity has increased significantly in China, with the prevalence of obesity increased from 4.0% in 2002 to 10.7% in 200914 and the prevalence of hypertension increasing from 18.8% in 200215 to 29.6% in 201416 in Chinese adults.
Obese patients with hypertension usually require more anti-hypertensive medications and have an increased risk of treatment-resistant hypertension10. In the past few years, the American Society of Hypertension, the Obesity Society, and the European Association for the Study of Obesity have published joint statements about obesity-related hypertension. However, little data has been published about the prevalence of obesity-related hypertension according to various world definitions.
Chengdu is one of the national centers for urban planning under the state council and is Southwest China’s science and technology, commercial, financial, traffic, and communication hub. Located in the upper Yangtze river region, Chongqing is one of the four municipalities directly under the central government and is also a commercial, financial, technical, innovative, and modern logistics center in Southwest China. In this study, we aimed to assess the prevalence of obesity-related hypertension in Southwest China, which is of value for the prevention and treatment of hypertension.
Materials and Methods
Study population
From September 2013 to March 2014, a multi-stage, stratified sampling was conducted on 10,589 people aged 40 to 79 years living in the urban communities of Chengdu and Chongqing investigated by using a questionnaire and performing physical and biochemical measurements. In the first phase of this study, the Jinjiang, Longquan, and Chenghua districts were randomly selected from the urban area of Chengdu; the Yubei and Jiangbei districts were randomly selected in Chongqing. In the second phase, a random sub-district was selected from each major district. In the third stage, one community was randomly selected from each sub-district, and a total of five random communities were selected. This study protocol was approved by the ethics committee of the Second People’s Hospital of Chengdu (NO 2013015), the methods in the study were in accordance with relevant guidelines, and a written informed consent was obtained from all participants.
Inclusions and exclusions
Residents aged 40–79 years who had lived in the selected communities for more than five years were included in the study. People with a history of secondary hypertension, mental illness, malignancies, renal failure requiring dialysis, or who refused to participate in this inquiry were excluded. From September 2013 to March 2014, 11,385 people were invited to participate. Due to missing sex, blood pressures, weight, waist circumference (WC), or body-mass index (BMI) data, 796 patients were excluded. Thus, 10,589 patients were included in the final analysis.
Data collection
More than 30 investigators were trained for data collection. All subjects filled out the same on-site questionnaire, which included demographic characteristics, lifestyle risk factors, and personal and family history, according to the cardiovascular survey methods of World Health Organization (WHO)17. The questionnaire also included height, weight, WC, and blood pressure measurements. When measuring the height and weight, the subject needed to be barefoot and take off any hat, wearing only lightweight clothes. The BMI was calculated by weight (kg) and by height (meters) squared. Investigators measured the minimum circumference between the inferior margin of the ribcage and the iliac crest as the WC measurement18. After the study, the subjects took a five-minute seated rest, then standardized mercury sphygmomanometers were used to measure their sitting blood pressures. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded at the first appearance and disappearance of Korotkoff sounds. Two blood pressure readings were obtained and averaged.
Blood sample collection and laboratory tests
Venous blood was drawn after 12 hours of fasting. Blood glucose, lipids, and uric acid (UA) levels were assessed in all blood samples. Patients without a history of diabetes mellitus (DM) were tested using the oral glucose tolerance test (OGTT), wherein 75 g of glucose was dissolved in 300 ml of warm water and was administered orally within five minutes, and venous blood was drawn two hours later. The total cholesterol (TC), triglycerides (TG), and blood glucose levels were detected by enzymatic methods. High-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) levels were measured using a homogeneous method. Serum UA was measured by the phosphotungstic acid method.
Diagnostic standards
According to the recommendations from 2017 American College of Cardiology (ACC) Guidelines, hypertension was defined as an SBP ≥130 mmHg and/or DBP ≥80 mmHg, and/or being diagnosed with hypertension and currently under antihypertensive drug treatment19. DM was defined as a fasting plasma glucose (FPG) level ≥7.0 mmol/L, 2-hour postprandial glucose (2-hPG) level ≥11.1 mmol/L, or a previous clinical diagnosis20. Overweight was defined as a BMI between 24.0 and 27.9 kg/m2. Obesity was defined as a BMI of 28.0 kg/m2 or more. Central obesity was defined as a WC of 90 cm or more in men and 85 cm or more in women21,22. According to the consensus of Chinese experts on obesity-related hypertension management and ACC guidelines, obesity-related hypertension was defined as a BMI of 28.0 kg/m2 or more and/or a WC of 90 cm or more in men and 85 cm or more in women, SBP ≥130 mmHg and/or DBP ≥80 mmHg, and/or being diagnosed with hypertension and currently under antihypertensive drug treatment. Participants with secondary hypertension were excluded from this study23. Hypertriglyceridemia was defined as a TG level ≥1.7 mmol/L. High LDL cholesterolemia was defined as a LDL-C level ≥3.4 mmol/L. Hypercholesterolemia was defined as a TC level ≥5.2 mmol/L based on the criteria of the NCEP Adult Treatment Panel III report24. A history of smoking was defined as smoking at least once per day for more than a year, and currently having smoked or quit smoking for less than three years. A history of drinking was defined as drinking at least once a week over a year, and currently having drunk or quit drinking for less than three years. The family history of hypertension was defined as immediate family members having hypertension. Physical exercise was defined as having at least one exercise session per week.
Statistical analysis
The Epidata3.1 software was used to double the input data to ensure their quality, and data processing and analysis were carried out using the SAS 9.2 software (Institute Inc. SAS, Cary, NC, USA). Quantitative data were compared using the one sample t-test or the Wilcoxon rank sum test, and qualitative data were compared using the χ2 test. The χ2 linear trend test was used to detect the trend in the prevalence of obesity-related hypertension in individuals in association with their age and BMI. Logistic regression was used to explore the potential risk factors for obesity-related hypertension. The covariates selected in the logistic regression model included age, sex, having or without spouse, education level, income, smoking, drinking, like to eat fried food (more than 3 times a week),family history of hypertension, daily main food more than 300 g/d or not, physical exercises, diabetes, hypertriglyceridemia, hypercholesterolemia, and hyperuricemia. A p-value of <0.05 was considered as significant.
Results
Prevalence of hypertension and obesity-related hypertension
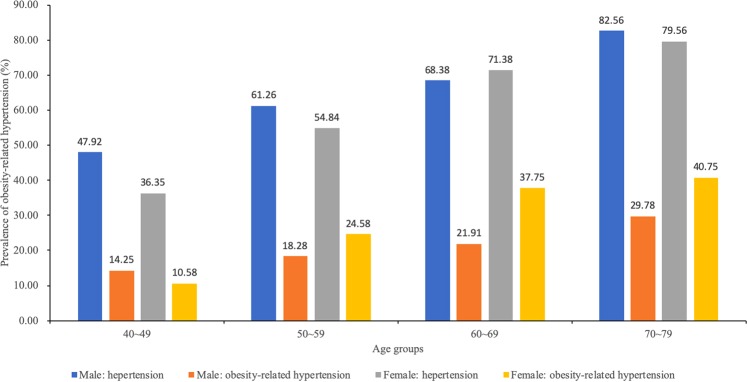
In this study, 10,589 adult participants aged 40 to 79 years in Southwest China were included, among whom 3,823 were men and 6,766 were women. The overall prevalence of hypertension was 57.4% (6,083/10,589), with obesity-related hypertension accounting for 39.6% (2,410/6,083) of hypertension cases and 22.8% (2,410/10,589) of all participants. Obesity-related hypertension was more common in women than in men (24.7% versus 19.4%, p < 0.001). In men, obesity-related hypertension accounted for 31.4% (742/2,365) of hypertensive patients, while in women, it accounted for 44.9% (1,668/3,718) of the hypertensive patients. There were differences in age, sex, marriage, and education between the four groups stratified by the presence or absence of obesity and hypertension (Table 1). The prevalence of hypertension and obesity-related hypertension increased with age (p < 0.001) in both sexes (Fig. 1). At age 70 and above, nearly 30% of men and 40% of women suffered from obesity-related hypertension. Participants without spouse had a higher prevalence of obesity-related hypertension (p < 0.001). The participants with lower education levels had a higher prevalence of obesity-related hypertension (p < 0.0001). There were no significant differences in the prevalence of obesity-related hypertension between the high-income and low-income groups.
Table 1.
Baseline characteristics of the study participants.
| Characteristics | Normal population (n = 3598) | Obesity without hypertension (n = 908) | Non-obesity-related hypertension (n = 3673) | Obesity-related hypertension (n = 2410) | P values |
|---|---|---|---|---|---|
| Sex | <0.0001 | ||||
| Male | 1227(34.1) | 231(25.4) | 1623(44.2) | 742(30.8) | |
| Female | 2371(65.9) | 677(74.6) | 2050(55.8) | 1668(69.2) | |
| Age, years | <0.0001 | ||||
| 40~49 | 1370(38.1) | 213(23.5) | 753(20.5) | 313(13.0) | |
| 50~59 | 1389(38.6) | 411(45.3) | 1411(38.4) | 926(38.4) | |
| 60~69 | 548(15.2) | 186(20.5) | 964(26.2) | 752(31.2) | |
| ≥70 | 127(3.5) | 82(9.0) | 478(13.0) | 396(16.4) | |
| Spouse | <0.0001 | ||||
| No | 255(7.1) | 72(7.9) | 342(9.3) | 257(10.7) | |
| Yes | 3318(92.2) | 825(90.9) | 3313(90.2) | 2141(88.8) | |
| Income | 0.26 | ||||
| ≤2000 yuan | 2825(78.5) | 716(78.9) | 2937(80.0) | 1906(79.1) | |
| >2000 yuan | 730(20.3) | 186(20.5) | 679(18.5) | 468(19.4) | |
| Education | <0.0001 | ||||
| middle school or below | 2510(69.8) | 709(78.1) | 2769(75.4) | 2009(83.4) | |
| High school education or above | 1073(29.8) | 196(21.6) | 875(23.8) | 388(16.1) |
Values are presented as number (%).
Figure 1.
The prevalence of hypertension and obesity-related hypertension across different age groups.
Characteristics of the obesity-related hypertensive and non-obesity-related hypertensive patients
The obesity-related hypertensive patients were older and had a higher BMI, WC, Hip circumference, SBP, TG, FPG, 2h-PG, UA, and LDL-C(all p < 0.05) and lower HDL-C (p < 0.05)(Table 2). There were no differences in height, DBP, heart rate (HR), or TC.
Table 2.
Baseline characteristics of the obesity-related hypertensive and non-obesity-related hypertensive patients.
| Variable | Overall | Non-obesity-related hypertension (N = 3673) | Obesity-related hypertension (N = 2410) | P † | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Men (N = 1623) | Women (N = 2050) | P* | Overall | Men (N = 742) | Women (N = 1668) | P* | |||
| Age, years | 57.81(29.43) | 58.72(18.90) | 59.11(10.18) | 58.41(23.62) | 0.231 | 62.52(38.29) | 62.74(42.1) | 62.42(36.48) | 0.859 | <0.001 |
| Height, cm | 157.3(11.89) | 157.02(8.50) | 163.08(6.19) | 152.23(6.85) | <0.001 | 157.13(8.72) | 165.18(7.47) | 153.54(6.57) | <0.001 | 0.638 |
| Weight, kg | 59.17(13.88) | 56.32(8.03) | 60.7(7.42) | 52.85(6.68) | <0.001 | 67.21(9.84) | 74.1(8.53) | 64.14(8.77) | <0.001 | <0.001 |
| BMI, kg/m2 | 23.83(3.42) | 22.78(2.38) | 22.81(2.34) | 22.76(2.41) | 0.543 | 27.14(3.10) | 27.11(2.68) | 27.16(3.27) | 0.716 | <0.001 |
| waist circumference, cm | 81.56(25.06) | 77.46(7.24) | 79.95(7.16) | 75.49(6.67) | <0.001 | 92.81(6.27) | 95.07(5.70) | 91.8(6.25) | <0.001 | <0.001 |
| Hip circumference, cm | 93.62(13.74) | 91.37(14.71) | 91.68(6.38) | 91.13(18.84) | 0.216 | 100.37(7.47) | 100.44(6.41) | 100.34(7.90) | 0.744 | <0.001 |
| systolic pressure, mmHg | 130.72(21.38) | 142.1(18.31) | 142.22(18.45) | 142.01(18.2) | 0.733 | 145.49(19.23) | 144.98(18.14) | 145.72(19.70) | 0.371 | <0.001 |
| Diastolic pressure, mmHg | 79.14(18.6) | 85.59(18.28) | 86(12.28) | 85.26(21.89) | 0.197 | 86.43(25.97) | 87.84(20.83) | 85.81(27.94) | 0.048 | 0.167 |
| Heart rate/min | 79.99(25.84) | 81.32(20.04) | 80.98(26.69) | 81.58(12.51) | 0.410 | 82.45(36.44) | 82.86(44.40) | 82.27(32.29) | 0.744 | 0.166 |
| TC, mmol/L | 4.63(0.93) | 4.69(0.94) | 4.57(0.90) | 4.79(0.97) | <0.001 | 4.76(0.91) | 4.59(0.88) | 4.84(0.91) | <0.001 | 0.05 |
| HDL-C, mmol/L | 1.42(0.36) | 1.43(0.32) | 1.38(0.34) | 1.47(0.30) | <0.001 | 1.33(0.31) | 1.24(0.29) | 1.37(0.31) | <0.001 | <0.001 |
| LDL-C, mmol/L | 2.52(0.76) | 2.55(0.77) | 2.48(0.77) | 2.61(0.77) | <0.001 | 2.63(0.77) | 2.6(0.79) | 2.65(0.76) | 0.166 | <0.001 |
| TG, mmol/L | 1.62(1.25) | 1.57(1.24) | 1.61(1.30) | 1.55(1.19) | 0.179 | 2.06(1.47) | 2.01(1.35) | 2.08(1.51) | 0.245 | <0.001 |
| FPG, mmol/l | 5.69(2.07) | 5.72(1.89) | 5.8(2.05) | 5.6 (1.75) | 0.02 | 6.14(2.34) | 5.94(1.77) | 6.23(2.54) | 0.001 | <0.001 |
| 2hPG, mmol/L | 7.91(3.83) | 7.99(3.69) | 7.88(3.87) | 8.08(3.54) | 0.131 | 9.29(4.46) | 8.71(3.63) | 9.56(4.77) | <0.001 | <0.001 |
| Uric acid, mmol/L | 291.8(83.26) | 293.59(83.96) | 336.54(86.12) | 259.87(64.62) | <0.001 | 315.26(89.47) | 376.5(86.96) | 288.4(76.4) | <0.001 | <0.001 |
Values are presented as mean (SD).
*p for comparison between men and women within either obesity-related hypertension or non-obesity-related hypertension.
†p for comparison between overall obesity-related hypertensive patients and non-obesity-related hypertensive patients.
Univariate analyses
Differences in the prevalence of cardiometabolic risk factors in obesity-related hypertensive and non-obese-related hypertensive subgroups are presented in Table 3. The obesity-related hypertensive patients had a higher prevalence of hypertriglyceridemia, diabetes, hyperuricemia and higher LDL-C (all p < 0.05). There was no difference in the prevalence of hypercholesterolemia (p = 0.60).
Table 3.
Prevalence of cardiovascular risk factors in the obesity-related hypertensive and non-obesity-related hypertensive patients.
| Variable | Overall | Non-obesity-related hypertension (N = 3673) | Obesity-related hypertension (N = 2410) | P † | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Men (N = 1623) | Women (N = 2050) | P* | Overall | Men (N = 742) | Women (N = 1668) | P* | |||
| Hypertriglyceridemia | 2207(36.3) | 1041(28.3) | 494(30.4) | 547(26.7) | 0.012 | 1166(48.4) | 352(47.4) | 814(48.8) | 0.537 | <0.001 |
| Hypercholesteremia | 788(13.0) | 469(12.8) | 154(9.5) | 315(15.4) | <0.001 | 319(13.2) | 59(8.0) | 260(15.6) | <0.001 | 0.595 |
| High low-density lipoprotein cholesterolemia | 756(12.4) | 427(11.6) | 142(8.7) | 285(13.9) | <0.001 | 329(13.7) | 90(12.1) | 239(14.3) | 0.147 | 0.019 |
| Diabetes mellitus | 1635(26.9) | 808(22.0) | 362(22.3) | 446(21.8) | 0.690 | 827(34.3) | 228(30.7) | 599(35.9) | 0.013 | <0.001 |
| Hyperuricemia | 799(13.1) | 347(9.4) | 217(13.4) | 130(6.3) | <0.001 | 452(18.8) | 200(26.9) | 252(15.1) | 0.022 | <0.001 |
Values are presented as number (%).
*p for comparison between men and women within either obesity-related hypertension or non-obesity-related hypertension.
†p for comparison overall obesity-related hypertensive patients and non-obesity-related hypertensive patients.
Multiple logistic regression
Multiple logistic regression analysis was used to determine the factors associated with obesity-related hypertension. The analysis showed that aging, current smoking, female gender, family history of hypertension, hypertriglyceridemia and diabetes were positively correlated with obesity-related hypertension. Higher education level and having spouse were negatively correlated with it. The results are presented in Table 4.
Table 4.
Multivariable-adjusted odds ratios for obesity-related hypertension.
| Characteristics | Odds Ratios (95% CI) | P |
|---|---|---|
| Female | 1.29 (1.03,1.62) | 0.025 |
| Age | 2.37 (1.63,3.23) | <0.0001 |
| High school education or above | 0.46(0.37, 0.57) | <0.0001 |
| Current smoking | 1.30(0.78, 1.98) | <0.0001 |
| Family history of hypertension | 1.29 (1.07, 1.56) | 0.008 |
| Hypertriglyceridemia | 1.68(1.05, 2.42) | <0.0001 |
| Diabetes mellitus | 1.80 (1.27, 2.43) | <0.0001 |
| Having spouse | 0.76 (0.58, 0.95) | 0.006 |
| Hyperuricemia | 2.01 (1.49, 2.69) | <0.0001 |
Age, sex, having or without spouse, education level, income, smoking, drinking, like to eat fried food (more than 3 times a week), family history of hypertension, family history of diabetes, daily main food more than 300 g/d or not, physical exercises, diabetes, hypertriglyceridemia, hypercholesterolemia, and hyperuricemia were treated as independent variables in the multifactorial logistic regression model.
Discussion
This is the first report about the prevalence of obesity-related hypertension among Chinese adults according to definitions set by 2017 ACC Guidelines, which is one of the study strong points. In this population-based investigation, we found that the prevalence of obesity-related hypertension and hypertension overall were 22.8% and 57.4%, respectively, among adults aged 40 to 79 years in Southwest China. This finding indicates that nearly 40% of hypertensive patients had obesity-related hypertension and that nearly 1 in 4 adults aged 40 to 79 years had obesity-related hypertension. Given the approximately 10% prevalence rate of obesity in China, the prevalence of obesity (~30%) and obesity-related hypertension (22.8%) were high among adults aged 40 to 79 years in Southwest China.
We showed that the prevalence of hypertension was higher in men than in women, similar to the previous studies15,16. In contrast, obesity-related hypertension was more prevalent in women (24.7%) than in men (19.4%). In this study, about 1/3 of men suffering from hypertension had obesity-related hypertension, whereas approximately 1/2 of women suffering from hypertension had it. The higher prevalence of obesity-related hypertension in women is partly due to the fact that women had nearly 1.4 times higher prevalence of obesity than men (34.7% versus 25.5%). Moreover, our study found that the prevalence of hypertension in obese participants was significantly higher than in non-obese participants (72.6% versus 50.5%). A prior study was done in 14 provinces of China also showed that the prevalence of obesity in women was higher than in men and the prevalence of hypertension was increased with BMI25, consistent with our observations. Other studies were done in China besides Southwest region have also shown a positive correlation between the prevalence of hypertension and BMI26,27. In order to achieve better control of hypertension in a community perspective, it is necessary to check blood pressures regularly in obese patients, especially those who are severely obese.
Some studies reported that a higher education level was associated with lower prevalence of hypertension28–30and that there was an inverse relationship between the educational level and obesity31–33. In our investigation, a higher education level was associated with a lower prevalence of obesity-related hypertension, while there were no significant differences between the high-income and the low-income groups. It has been suggested that in a period of economic development, it is of great importance to improve the population’s level of cultural knowledge, especially in terms of health, to improve the health of the population as a whole. Our study showed that unmarried people had a higher prevalence of both hypertension and obesity-related hypertension. Former studies reported that unmarried persons were positively correlated with hypertension and had an increased mortality from cerebrovascular diseases34–36. Previous research assumed that married people could improve their quality of life and medication compliance in geriatric patients with chronic illnesses37–39. We speculate that most of the middle-aged and elderly who have a spouse are emotionally more stable and eat more regularly, though further study is needed.
Previous studies have suggested that smoking leads to hypertension40,41, mainly because it stimulates the sympathetic nervous system and accelerates atherosclerosis42. Previous studies have also suggested that smoking leads to a decline in BMI and the prevalence of obesity43,44. One study suggested that cigarette smoking was positively associated with abdominal obesity45. Our study suggested that smoking may increase the prevalence of obesity-related hypertension and is a potential risk factor for it, which also requires further study.
A higher prevalence of hypertriglyceridemia, DM, and hyperuricemia was observed in the obesity-related hypertension group, which has been ascribed to be related to central obesity, insulin resistance, and subclinical inflammation46–49. Besides, osteoprotegerin, camellia sinensis extract, mineral metabolism, has been ascribed to be related to arteriosclerosis, insulin resistance, and lipids metabolism in hypertentsion50–53. In this study, approximately half of obesity-related hypertensive patients had hypertriglyceridemia and there were 1 diabetic patients in every 3 obesity-related hypertension patients, aged 40 to 79 years. Hypertension, obesity, and DM have become the leading causes of cardiovascular disease and death in many developing countries54–56, and a higher UA level has been associated with an increased risk of hypertension57,58. In addition, we found that the prevalence of high LDL-C was higher in obesity-related hypertensive patients. Compared to the non-obesity-related hypertensive patients, regular screenings for cardiovascular metabolic risk factors in obesity-related hypertensive patients should be advocated.
The strengths of this study include a large number of community-dwelling participants included in this survey and a detailed report of hypertension and obesity-related hypertension based on the 2017 ACC Guidelines in Southwest China. Our study has several limitations. First, this study was a cross-sectional study, meaning that the findings cannot be used to establish a conclusive cause-and-effect relationship between risk factors and obesity-related hypertension. Second, the participants were recruited from Southwest China, such that the conclusions cannot represent the situation in other regions of China. Third, we did not perform detailed body composition analysis due to constraints of budgets.
Conclusions
The prevalence of obesity-related hypertension was high in people aged 40 to 79 years in Southwest China. Its prevalence was higher in women than in men and increased with age. Additionally, patients with obesity-related hypertension had a higher prevalence of DM, hypertriglyceridemia, high low-density lipoprotein cholesterolemia and hyperuricemia than non-obesity-related hypertensive patients. Aggressive strategies aiming at the prevention and treatment of obesity-related hypertension are needed.
Acknowledgements
This research was supported by the Szechwan Province Science and Technology Agency Fund Project (2009FZ 0027), Chengdu, China and Population and health project of Chengdu Municipal Science and Technology Bureau (10YTYB272SF-182), Chengdu, China.
Author contributions
X.B.H., T.D.W. put forward key ideas, objectives and goals. F.X., W.W.T. and L.S.H. participated in the design of this study and performed the statistical analysis. X.B.H., J.X.L., Y.L., Y.J.Y., T.S.H. and R.H. participated in the data collection and checked the data. Y.Z., L.S.H., X.L., X.B.H., T.D.W. and R.H.X. wrote and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yang Zhang, Li-Sha Hou and Wei-Wei Tang.
Contributor Information
Tzung-Dau Wang, Email: tdwang@ntu.edu.tw.
Xiao-Bo Huang, Email: drhuangxiaobo@126.com.
References
- 1.James PA, et al. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Corrigan SA, Raczynski JM, Swencionis C, Jennings SG. Weight reduction in the prevention and treatment of hypertension: a review of representative clinical trials. Am J Health Promot. 1991;5:208–214. doi: 10.4278/0890-1171-5.3.208. [DOI] [PubMed] [Google Scholar]
- 4.Peeters A, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MD, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Alagiakrishnan K, Banach M, Ahmed A, Aronow WS. Complex relationship of obesity and obesity paradox in heart failure - higher risk of developing heart failure and better outcomes in established heart failure. Ann Med. 2016;48:603–613. doi: 10.1080/07853890.2016.1197415. [DOI] [PubMed] [Google Scholar]
- 7.Berrington DGA, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock G, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 10.Jordan J, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens. 2012;30:1047–1055. doi: 10.1097/HJH.0b013e3283537347. [DOI] [PubMed] [Google Scholar]
- 11.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23:1170–1178. doi: 10.1038/ajh.2010.172. [DOI] [PubMed] [Google Scholar]
- 12.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100:191–199. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol. 2006;100:481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 14.Xi B, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev. 2012;13:287–296. doi: 10.1111/j.1467-789X.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from the China National Nutrition and Health Survey 2002. Circulation. 2008;118:2679–2686. doi: 10.1161/CIRCULATIONAHA.108.788166. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhang L, Wang F, Liu L, Wang H. Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens. 2014;27:1355–1361. doi: 10.1093/ajh/hpu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 18.Zhang S, et al. Genetic and environmental contributions to phenotypic components of metabolic syndrome: a population-based twin study. Obesity (Silver Spring) 2009;17:1581–1587. doi: 10.1038/oby.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 20.L’Heveder R, Nolan T. International Diabetes Federation. Diabetes Res Clin Pract. 2013;101:349–351. doi: 10.1016/j.diabres.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579–615. [PubMed] [Google Scholar]
- 22.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 23.Chinese expert consensus on obesity-related hypertension management. Zhonghua Xin Xue Guan Bing Za Zhi44, 212–219 (2016). [DOI] [PubMed]
- 24.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama285, 2486–2497 (2001). [DOI] [PubMed]
- 25.Chen J, Zhao XL, Wu F, Cui YL, Hu DY. Epidemiology of obesity and overweight and relation thereof to the prevalence of hypertension in 14 provinces/municipality in China. Zhonghua Yi Xue Za Zhi. 2005;85:2830–2834. [PubMed] [Google Scholar]
- 26.Zhang L, Zhang WH, Zhang L, Wang PY. Prevalence of overweight/obesity and its associations with hypertension, diabetes, dyslipidemia, and metabolic syndrome: a survey in the suburban area of Beijing, 2007. Obes Facts. 2011;4:284–289. doi: 10.1159/000331014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, et al. Obesity, emerging risk in China: trend of increasing prevalence of obesity and its association with hypertension and hypercholesterolaemia among the Chinese. Clin Exp Pharmacol Physiol. 2004;31(Suppl 2):S8–S10. doi: 10.1111/j.1440-1681.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- 28.Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–229. doi: 10.1097/HJH.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 29.Kautzky-Willer A, Dorner T, Jensby A, Rieder A. Women show a closer association between educational level and hypertension or diabetes mellitus than males: a secondary analysis from the Austrian HIS. Bmc Public Health. 2012;12:392. doi: 10.1186/1471-2458-12-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erceg M, Ivicevic-Uhernik A, Kern J, Vuletic S. Is there any association between blood pressure and education level? The CroHort study. Coll Antropol. 2012;36(Suppl 1):125–129. doi: 10.5671/ca.2012361s.125. [DOI] [PubMed] [Google Scholar]
- 31.Newton S, Braithwaite D, Akinyemiju TF. Socio-economic status over the life course and obesity: Systematic review and meta-analysis. Plos One. 2017;12:e177151. doi: 10.1371/journal.pone.0177151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boylan SM, Gill TP, Hare-Bruun H, Andersen LB, Heitmann BL. Associations between adolescent and adult socioeconomic status and risk of obesity and overweight in Danish adults. Obes Res Clin Pract. 2014;8:e163–e171. doi: 10.1016/j.orcp.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Heraclides A, Brunner E. Social mobility and social accumulation across the life course in relation to adult overweight and obesity: the Whitehall II study. J Epidemiol Community Health. 2010;64:714–719. doi: 10.1136/jech.2009.087692. [DOI] [PubMed] [Google Scholar]
- 34.Singh, M. et al. Prevalence and correlates of hypertension in a semi-rural population of Southern India. J Hum Hypertens (2017). [DOI] [PMC free article] [PubMed]
- 35.Tang Z, et al. Risk factors for cerebrovascular disease mortality among the elderly in Beijing: a competing risk analysis. Plos One. 2014;9:e87884. doi: 10.1371/journal.pone.0087884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu S, et al. Influence of life-related factors and participation in health examination on mortality in a 4.5-year follow-up of a rural cohort. Environ Health Prev Med. 2000;5:66–71. doi: 10.1007/BF02932006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah VR, Christian DS, Prajapati AC, Patel MM, Sonaliya KN. Quality of life among elderly population residing in urban field practice area of a tertiary care institute of Ahmedabad city, Gujarat. J Family Med Prim Care. 2017;6:101–105. doi: 10.4103/2249-4863.214965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shruthi R, Jyothi R, Pundarikaksha HP, Nagesh GN, Tushar TJ. A Study of Medication Compliance in Geriatric Patients with Chronic Illnesses at a Tertiary Care Hospital. J Clin Diagn Res. 2016;10:C40–C43. doi: 10.1111/crj.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han MA, et al. Health-related quality of life assessment by the EuroQol-5D in some rural adults. J Prev Med Public Health. 2008;41:173–180. doi: 10.3961/jpmph.2008.41.3.173. [DOI] [PubMed] [Google Scholar]
- 40.Hu W, et al. Association between cigarette smoking and hypertension in men: a dose response relationship analysis. Zhonghua Xin Xue Guan Bing Za Zhi. 2014;42:773–777. [PubMed] [Google Scholar]
- 41.Feng D, et al. The association between smoking quantity and hypertension mediated by inflammation in Chinese current smokers. J HYPERTENS. 2013;31:1798–1805. doi: 10.1097/HJH.0b013e328362c21a. [DOI] [PubMed] [Google Scholar]
- 42.Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16:2518–2525. doi: 10.2174/138161210792062920. [DOI] [PubMed] [Google Scholar]
- 43.Ginawi, I. A. et al. Association Between Obesity and Cigarette Smoking: A Community-Based Study. 6, 149–153 (2016).
- 44.Kayhan, S. The relationship between cigarette smoking and obesity. Journal of Experimental & Clinical Medicine (2013).
- 45.Clair C, et al. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: cross-sectional data from a population-based survey. Bmc Public Health. 2011;11:23. doi: 10.1186/1471-2458-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepien M, et al. Predictors of insulin resistance in patients with obesity: a pilot study. Angiology. 2014;65:22–30. doi: 10.1177/0003319712468291. [DOI] [PubMed] [Google Scholar]
- 47.Stepien M, et al. Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: a comparative pilot study. Lipids Health Dis. 2014;13:29. doi: 10.1186/1476-511X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghomari-Boukhatem H, et al. Blood pressure, dyslipidemia and inflammatory factors are related to body mass index in scholar adolescents. Arch Med Sci. 2017;13:46–52. doi: 10.5114/aoms.2017.64713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soran H, et al. Hypercholesterolaemia - practical information for non-specialists. Arch Med Sci. 2018;14:1–21. doi: 10.5114/aoms.2018.72238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suliburska Joanna, Skrypnik Katarzyna, Szulińska Monika, Kupsz Justyna, Markuszewski Leszek, Bogdański Paweł. Diuretics, Ca-Antagonists, and Angiotensin-Converting Enzyme Inhibitors Affect Zinc Status in Hypertensive Patients on Monotherapy: A Randomized Trial. Nutrients. 2018;10(9):1284. doi: 10.3390/nu10091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stepien, M. et al. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemia, and insulin resistance in an NaCl-induced hypertensive rat model. J Physiol Pharmacol69 (2018). [DOI] [PubMed]
- 52.Suliburska J, Skrypnik K, Szulinska M, Kupsz J, Bogdanski P. Effect of hypotensive therapy combined with modified diet or zinc supplementation on biochemical parameters and mineral status in hypertensive patients. J Trace Elem Med Biol. 2018;47:140–148. doi: 10.1016/j.jtemb.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Musialik K, Szulinska M, Hen K, Skrypnik D, Bogdanski P. The relation between osteoprotegerin, inflammatory processes, and atherosclerosis in patients with metabolic syndrome. Eur Rev Med Pharmacol Sci. 2017;21:4379–4385. [PubMed] [Google Scholar]
- 54.He J, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 55.Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350:2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 56.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.CIR.97.6.596. [DOI] [PubMed] [Google Scholar]
- 57.Wu L, et al. Association between serum uric acid level and hypertension in a Chinese elderly rural population. Clin Exp Hypertens. 2017;39:505–512. doi: 10.1080/10641963.2016.1259325. [DOI] [PubMed] [Google Scholar]
- 58.Han GM, Gonzalez S, DeVries D. Combined effect of hyperuricemia and overweight/obesity on the prevalence of hypertension among US adults: result from the National Health and Nutrition Examination Survey. J Hum Hypertens. 2014;28:579–586. doi: 10.1038/jhh.2014.31. [DOI] [PubMed] [Google Scholar]