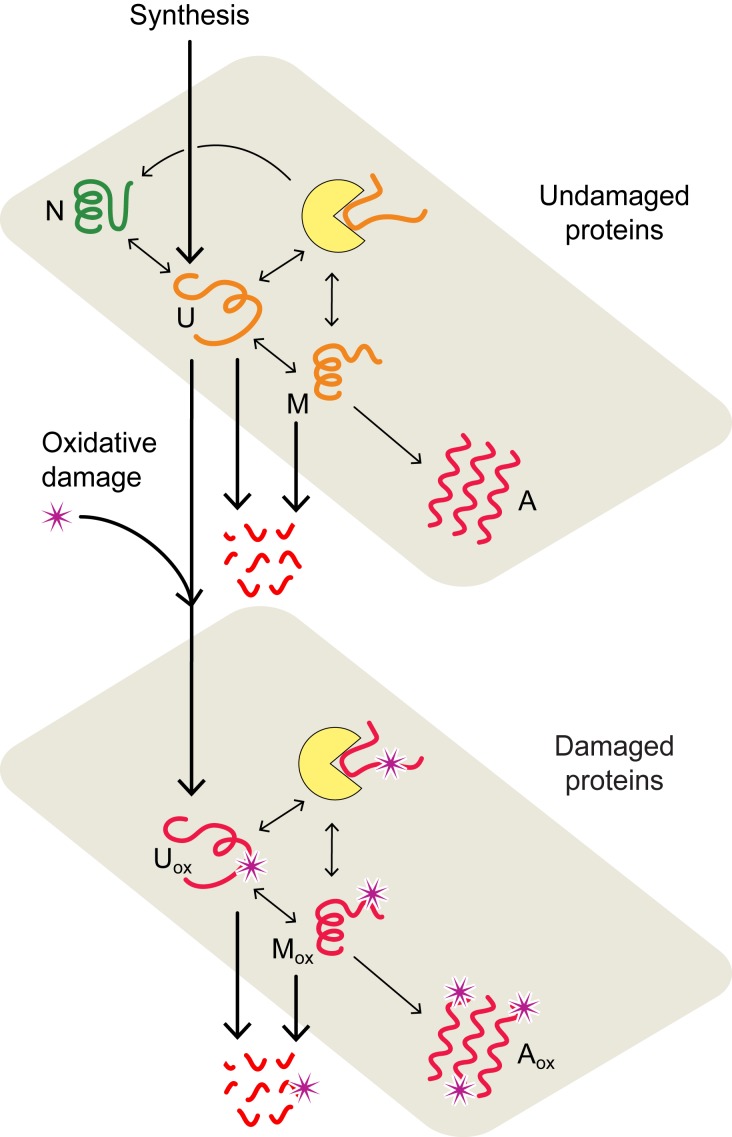
Fig. 1.
Proteostasis model of cell aging. In the model, proteins convert between native (N), unfolded (U), misfolded (M), and aggregated (A) states (upper plane) with rates estimated from experimental data (33, 42). Chaperone-dependent folding of newly synthesized or thermally unfolded proteins compete with the processes of protein oxidation, aggregation, and degradation, collectively defining the quality of the proteome and proteostasis at a given age. The lower plane describes the interconversion of oxidatively damaged unfolded (), misfolded (), and aggregated () states. Chaperones are represented by yellow disks.