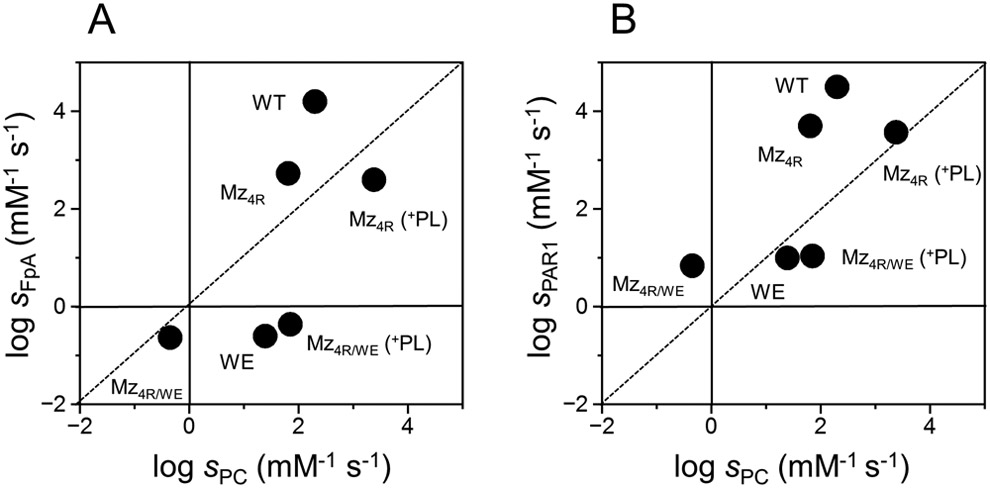
Figure 2A-B.
Two dimensional plots of Mz and thrombin activities. Values of s=kcat/Km for cleavage of (A) fibrinogen (sFpA) or (B) PAR1 (sPAR1) are plotted vs those for protein C (sPC) activation. The region below the diagonal dotted line denotes activity toward protein C that exceeds that for fibrinogen (A) or PAR1 (B). The reverse is true for the region above the diagonal line. Phospholipids (PL) at a saturating concentration of 0.5 mM (see also Figure 3) selectively enhance the activity of Mz (Mz4R or Mz4R/WE) toward protein C, without affecting the rates of fibrinogen and PAR1 cleavage. The WE mutation profoundly decreases the activity of thrombin (WE) and Mz (Mz4R/WE) toward fibrinogen and PAR1. Addition of phospholipids completely restores the activity of Mz4R/WE toward protein C and produces a significant anticoagulant effect that slightly exceeds that of the thrombin mutant WE. Values for wild-type (WT) and WE (WE) thrombin are from ref. [45].