Abstract
Background
Right ventricular longitudinal strain (RVLS) has emerged as an approach for quantifying RV function in diseases such as pulmonary hypertension (PH) and congenital heart disease. A major limitation in applying RVLS is that strain imaging and analysis is proprietary, which may result in systematic differences from vendor to vendor. The goal of this study was to test the reproducibility of RV strain analysis between selected vendor-specific and vendor-independent software packages on images obtained from different ultrasound scanners, as would be common in clinical practice or in a multicenter clinical trial.
Methods
In this prospective, single center study, thirty-five patients (five healthy subjects and thirty with PH) each received two echocardiographic scans, one using GE (Vivid E9) and the other using Philips (iE33) ultrasound systems. Images were analyzed using both vendor-specific (VSS) and vendor-independent (TomTec VIS) software for determination of RVLS. A repeated measures analysis of variance (ANOVA) was used to assess for any systematic differences between methods, as well as effects of scanner and software and a possible interaction between scanner and software for each strain measurement.
Results
Differences for global strains were not statistically significant between VSSs (p≥0.05) but some differences were noted between VSS and VIS. Wide variability between regional peak strain measurements was noted but no systematic differences were found.
Conclusion
Global RVLS values between VSS systems are not significantly different but may differ slightly from VIS. When comparing regional strains between VSS and VIS analyses, there is widespread variability without clear systematic differences.
Keywords: right ventricular strain, echocardiography, reproducibility, validation
Introduction
The ability to accurately and reproducibly measure right ventricular (RV) function has been of great interest both from clinical and research perspectives1–4, as RV functional impairment has been associated with negative outcomes in diseases such as pulmonary hypertension (PH)3, 5–7 and congenital heart disease8, 9. RV function may be assessed by invasive methods such as cardiac catheterization. Non-invasively, the “gold standard” method is currently cardiac magnetic resonance imaging (MRI)10 which is often limited by patient tolerability and institutional availability.11
Transthoracic echocardiography (TTE) is, however. is a widely available imaging method that, accordingly, provides ample opportunity to evaluate patients with diseases that may affect RV function. Problematically, most standard Doppler echo-derived parameters, such as pulmonary artery (PA) systolic pressure (estimated from tricuspid regurgitation, TR) or RV chamber dimensions do not provide a direct measure of RV function.4 More advanced quantitative measures of RV function, such as tricuspid annulus systolic excursion (TAPSE), RV fractional area change (FAC) have geometric assumptions. These, along with the RV Tei index, and have not been validated in large trials.4
RV peak systolic echo speckled tracked echo (2DSTE) longitudinal strain (RVLS) has emerged as an approach for quantifying RV function. RVLS provides more global assessment of RV function12 and has relative angle-independence.13 Moreover, RVLS has been associated with outcomes in PH3, 6 and other diseases that affect RV function8, 14, suggesting that it could be used as a standard and reproducible approach to quantify RV function.
Despite these advantages, there are still potential limitations to the application of RVLS. Echocardiographic strain imaging and image analysis methods are mainly proprietary (vendor-specific) and subject to variations.15, 16 While such differences have been studied in left ventricular (LV) longitudinal strain and shown not to be significant16–18, this may not be directly applicable to the RV.19
Currently, the reproducibility of RV strain across different vendor-specific software (VSS) and vendor-independent software (VIS) platforms (all using different algorithms to calculate 2DSTE strain) has not been validated. With its complex geometry and different orientation of myocardial fibers compared to the LV19, it is unclear whether these varied algorithms for strain would yield similar values for RVLS. Different methodologies may result in systematic differences of RV strain between study intervals when the same systems are not used, limiting the clinical and research applicability of RV GLS.
The goal of this study was to investigate the agreement and reproducibility of RV strain measurements between VSS and a single VIS package on images obtained from different ultrasound machines.
Methods
Study Population
In this prospective study, patients sent by their referring provider for a clinically indicated echocardiogram were recruited from the Duke University Medical Center Echo Lab. Patients were included if they were adults able to provide consent. Exclusion criteria included: Poor imaging windows or image quality that precluded strain analysis (i.e., the walls of the RV apical four chamber view were not adequately visible throughout the cardiac cycle and/or two or more wall segments (adjacent or not) were not tracked during the cardiac cycle); arrhythmia (defined as atrial fibrillation/atrial arrhythmias or one or more PVCs within a 3 beat loop), or the presence of significant congenital heart disease (i.e. large ventricular septal defect or complex cardiac defects such as transposition or single ventricle).
Patient characteristics were recorded and presented as median, 25th, and 75th percentiles for continuous variables, and frequencies and percentages for categorical variables. Characteristics were assessed based on the health of the subject (healthy or PH) and on the total study group.
Study Design
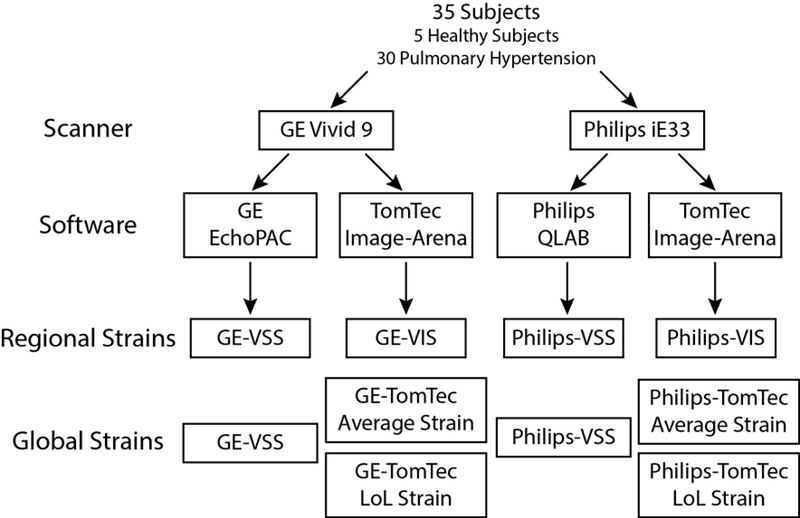
Each patient received two scans, one using a GE Vivid 9 and the other using a Philips iE33 scanner (Fig. 1). The order of scanner use was random, depending on which device was available for the initial clinically-indicated echocardiogram. After the first scan was complete, a second scan was performed on a different ultrasound machine by the same sonographer within 60 minutes. All sonographers were experienced in obtaining and optimizing images for strain analysis.
Figure 1. Study design for comparing RV regional and global strains across different scanners and software.
Thirty-five subjects had images obtained on two scanners (GE and Philips) followed by analysis in vendor-specific (VSS) and independent (VIS) software, yielding regional and global strains (see text for full details).
For this study, three previously described RV-focused apical views, were obtained by rotation of the ultrasound probe by 60 degrees around the apex of the right ventricle instead of the left ventricle.20 This approach was developed and validated in this laboratory to provide a comprehensive examination of the right ventricle as it allows the use of LV strain software for RV analysis. Using this approach, the AP4 position views the RV lateral free wall and septum (except it is mirrored), the AP2 rotational position, visualizes the posterior RV free wall, anterior septum and outflow tract; and the AP3 view visualizes the anterior free wall, posterior septum and the RV inflow.
Images were then analyzed using both vendor-specific (VSS) and vendor-independent software (VIS), yielding two strain analysis sets for each examination encounter (VSS and VIS for each GE and Philips study) for a total of 4 sets of regional strain measurements for each subject (GE-VSS, GE-VIS, Philips-VSS and Philips-VIS). For evaluating global strain, two calculation methods available in the VIS TomTec software (Average and Length of Line (LoL)) were applied, yielding a total of six measures of global strain per subject (Fig. 1).
Strain Analysis
Echocardiographic studies were performed on GE Vivid E9 using a 3.5 MHz probe (GE Vingmed Ultrasound, Horten, Norway) and a Philips IE33 (Philips Ultrasound, Andover, Mass. For this study, the comprehensive three RV-focused apical views were obtained with breath hold in three beat loops and optimized in depth for strain analysis (with frame rates between 45 and 90 Hz).2, 12 Off-line analysis of GE images were performed by EchoPAC version BT13 (GE Vingmed Ultrasound AS, Horten, Norway), Philips images by on cart QLAB version 10.4 (Philips, Andover, MA) and both GE and Philips images by off-line Image-Arena, 2D CPA version 1.2 (TomTec Imaging Systems, Unterschleissheim, Germany) by a single experienced reader and analysis was confirmed by a separate experienced reader. While inter-reader variability for these studies have been shown to be low2 such possible inter- and intra-reader variability between two expert readers was determined in this study using the coefficients of variation (CoV) for global VIS from echos from ten subjects.
Right ventricular strain measurements for both global and regional strain types were calculated using VSS (EchoPAC and QLAB) and VIS (TomTec). Both VSS approaches are based on speckle-tracking strain measurements from the mid-myocardium.15 VIS software allowed calculation of regional and global speckle tracked strain where “Average” strain was the peak average of regional segments from the endocardium and GLS was determined from the length of an endocardial line (which we refer to as “LoL” for length-of-line) strain. An average of the 2-, 3-, and 4-chamber global measurements was calculated for both types of global strain (Average and Length of Line). All attempts were made to use the same beat used for VSS with VIS.
Subject Population and Power Calculation
Thirty-five subjects were enrolled in the study: five healthy controls and thirty subjects with pulmonary hypertension. Healthy controls were identified as subjects who had structurally normal heart who had clinically ordered echocardiograms who, upon review, did not possess structural abnormalities. Patients with a diagnosis of PH all had the ICD-9 code of 416.8 and/or the primary diagnosis of PH as listed in their clinic note. Given the variability in global strain measurements that was observed, with 35 subjects the study was powered to detect differences between strain values at or greater than 2.0% and standard deviation of the bias (SDb) less than 4%, or smaller differences (<2.0%) with little variability (SDb < 2.0%). With 30 subjects (i.e. PH subjects), we are powered to detect differences at or greater than 2.5 and SDb less than 5, or a difference of 2.0% with little variability (SDb < 3.0%).
Statistical Analysis
The main analysis of the study was to determine agreement between strain measurements obtained using different scanners and software. Agreement was assessed between images from different scanners by VIS, between the different VSS, and finally between VSS and VIS for each scanner. For each comparison, the observed difference in measurements was calculated for each patient to describe bias (method 1 - method 2). Both the overall mean and standard deviation (calculated from the average measurements for each patient) and the mean and SDb were calculated.
The 95% confidence limits of agreement were determined by finding 2 standard deviations (SDb) above and below the mean bias. The coefficient of variation (CoV) was calculated for each pair of methods as the ratio of (SDb/sqrt(2)) to the absolute value of the overall mean, expressed as a percent. CoV quantifies the amount of variability that we would expect to see between replicates relative to the mean. Since we did not have replicate measurements within each method to evaluate between-replicate variation for a particular method, the CoV can be used to estimate this variability, but assumes that variability is similar for the two methods being compared after systematic differences are removed. A repeated measures analysis of variance (ANOVA) was used to assess for any systematic differences between methods, as well as main (i.e., marginal) effects of scanner and software and a possible interaction between scanner and software for each strain measurement. Significance of the bias between software (VIS vs. VSS) within each scanner, and of the bias between scanners within each software package was determined using specific contrasts calculated from the same ANOVA models.
Bland-Altman plots of agreement were created for the global right ventricle strain of each of the different chamber views for comparison. These plots examined differences between software and scanner combinations by plotting the percent difference against the average of the two scanner/software combinations being assessed. Reference lines indicating the average percent bias and the 95% limits of agreement were displayed on the plots. A red reference line indicating 0 bias was also included. Measures of global right ventricle strain had low incidence of missing data. Regional strain measures were slightly less complete, with extent of missing data indicated in tables. Calculations were based on all available information with no imputation of missing values. P-values < 0.05 were interpreted as statistically significant.
Given the goals of the analysis (to characterize differences between software and scanners), we did not attempt to control the significance level for multiple comparisons. As such, the chance of finding at least one significant difference is greater than the nominal significance level of 0.05. However, all of the analyzed comparisons are reported in this manuscript and the probability of a Type I error occurring in any individual test is 0.05. Analyses were generated using SAS v 9.4 (SAS Institute, Cary NC).
Results
Baseline characteristics
In total, thirty-five subjects were recruited for the study: five healthy controls and thirty subjects with PH (Table 1). There were a higher proportion of females (77.1%), consistent with a PH population.21 The median age was 60 years in PH subjects compared to 45 years in the healthy subjects, and 43% were African American overall.
Table 1.
Baseline subject characteristics (counts and percentage, or median with interquartile range in parenthesis)
| Demographic | Healthy Subjects (N=5) | Pulmonary Hypertension Subjects (N=30) | All Subjects (N=35) |
|---|---|---|---|
| Age (years) | |||
| Median (25th, 75th) | 45.0 (44, 67) | 59.5 (51, 70) | 58.0 (49, 70) |
| Race | |||
| African American | 0 (0.0%) | 15 (50.0%) | 15 (42.9%) |
| Caucasian | 5 (100.0%) | 15 (50.0%) | 20 (57.1%) |
| Gender | |||
| Female | 3 (60.0%) | 24 (80.0%) | 27 (77.1%) |
| Male | 2 (40.0%) | 6 (20.0%) | 8 (22.9%) |
Inter-Reader Variability
Inter-reader variability was 9.6% [3.4%, 17.3%] (median [IQR]) and intra-reader variability was 8.5% [3.1%, 15.7%]. This degree of variability was consistent with that observed in a previous study of LV strain from this laboratory.16
Assessment of Scanner and Software Effects
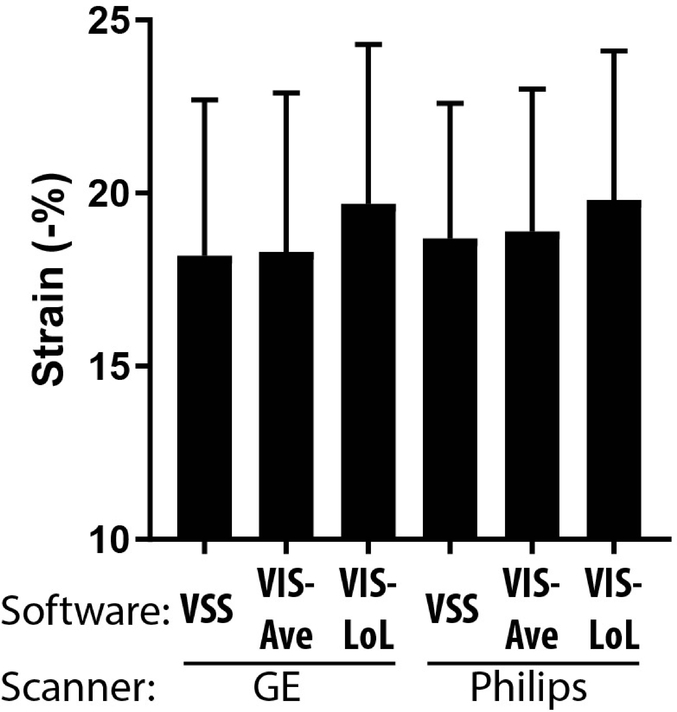
Each subject was scanned followed by VSS and VIS determination of RV strain from three views as indicated in Methods (Fig. 1). These sets of strain values were analyzed by ANOVA to understand generalized interactions between software and scanner effects (Supplementary Table 1), and were categorized into both global and regional strain values. Wide variability was present throughout all strain values, but was especially noted in regional values. Right ventricular strain measurements for both global and regional strain types calculated using VSS and VIS (Table 2a and Table 2b for global and regional strain measurements, respectively). For global strains (Table 2a), TomTec LoL measures were on average ~1.5–2% higher, i.e., more negative, than VSS measures for 2 and 4 chamber views. Differences in the average strain of the 2-, and 4-chamber views were statistically significant, with TomTec LoL tending to give slightly higher (more negative) measures, with slightly larger differences between LoL and other methods observed for GE. For regional strains (Table 2b), there was wide variability including significant differences in multiple views. However, no appreciable pattern or systematic influence was identified.
Table 2a.
Scanner and software global RV longitudinal strains for 2, 3 and 4 chamber views. Strain values are in %.
| Variables | GE VSS | GE - VIS Average Strain | GE - VIS LoL Strain | Philips VSS | Philips - VIS Average Strain | Philips – VIS LoL Strain | P-value |
|---|---|---|---|---|---|---|---|
| 2 Chamber | |||||||
| N (N missing) | 35 (0) | 35 (0) | 35 (0) | 30 (5) | 34 (1) | 34 (1) | <.0001 |
| Mean (SD) | −18.1 (5.7) | −18.1 (5.3) | −20.2 (5.5) | −19.0 (4.4) | −18.9 (4.9) | −20.1 (4.9) | |
| 3 Chamber | |||||||
| N (N missing) | 35 (0) | 35 (0) | 35 (0) | 28 (7) | 34 (1) | 34 (1) | 0.52 |
| Mean (SD) | −18.3 (4.5) | −18.2 (4.8) | −18.8 (5.5) | −18.5 (4.5) | −18.0 (4.3) | −19.1 (5.0) | |
| 4 Chamber | |||||||
| N (N missing) | 35 (0) | 35 (0) | 35 (0) | 32 (3) | 35 (0) | 35 (0) | <.0001 |
| Mean (SD) | −18.4 (4.4) | −18.4 (4.4) | −20.0 (4.5) | −18.9 (4.1) | −18.9 (4.1) | −20.3 (4.5) | |
| Average | |||||||
| N (N missing) | 35 (0) | 35 (0) | 35 (0) | 34 (1) | 35 (0) | 35 (0) | <.0001 |
| Mean (SD) | −18.2 (4.5) | −18.3 (4.6) | −19.7 (4.6) | −18.7 (3.9) | −18.9 (4.1) | −19.8 (4.3) |
LOL= length of line; VSS=vendor-specific software
Table 2b.
Scanner and software regional RV longitudinal strains from multiple RV views (see text for details). Strain values are in %.
| Variables | GE - VSS | GE - VIS | Philips - VSS | Philips - VIS | P-value |
|---|---|---|---|---|---|
| 2 Chamber | |||||
| Apical Anterior Strain | |||||
| N (N missing) | 33 (2) | 34 (1) | 27 (8) | 32 (3) | |
| Mean (SD) | −17.6 (9.2) | −17.2 (8.3) | −25.0 (6.1) | −19.0 (9.7) | <.0001 |
| Apical Inferior Strain | |||||
| N (N missing) | 33 (2) | 33 (2) | 30 (5) | 32 (3) | |
| Mean (SD) | −23.4 (8.1) | −20.8 (8.2) | −20.7 (5.7) | −24.4 (7.3) | 0.01 |
| Basal Anterior Strain | |||||
| N (N missing) | 35 (0) | 32 (3) | 30 (5) | 32 (3) | |
| Mean (SD) | −13.0 (6.6) | −16.3 (7.5) | −16.2 (5.0) | −17.9 (5.7) | <.01 |
| Basal Inferior Strain | |||||
| N (N missing) | 33 (2) | 34 (1) | 28 (7) | 32 (3) | |
| Mean (SD) | −17.7 (6.2) | −17.8 (9.1) | −15.1 (4.9) | −18.1 (8.3) | 0.03 |
| Mid Anterior Strain | |||||
| N (N missing) | 35 (0) | 35 (0) | 29 (6) | 34 (1) | |
| Mean (SD) | −14.3 (6.2) | −18.1 (8.3) | −14.6 (4.2) | −18.6 (6.0) | <.0001 |
| Mid Inferior Strain | |||||
| N (N missing) | 34 (1) | 35 (0) | 30 (5) | 31 (4) | |
| Mean (SD) | −21.1 (6.5) | −20.2 (7.8) | −21.0 (5.7) | −20.1 (7.8) | 0.73 |
| 3 Chamber | |||||
| Apical Anterior Strain | |||||
| N (N missing) | 32 (3) | 33 (2) | 27 (8) | 32 (3) | |
| Mean (SD) | −16.8 (8.4) | −21.0 (8.0) | −24.0 (6.6) | −20.3 (8.7) | <.001 |
| Apical Posterior Strain | |||||
| N (N missing) | 32 (3) | 31 (4) | 26 (9) | 32 (3) | |
| Mean (SD) | −16.8 (9.2) | −17.4 (10.7) | −19.8 (7.0) | −14.8 (10.7) | 0.04 |
| Basal Anteroseptal Strain | |||||
| N (N missing) | 35 (0) | 35 (0) | 25 (10) | 34 (1) | |
| Mean (SD) | −16.2 (5.2) | −18.0 (6.8) | −15.1 (4.8) | −15.8 (6.2) | 0.20 |
| Basal Posterior Strain | |||||
| N (N missing) | 29 (6) | 28 (7) | 25 (10) | 26 (9) | |
| Mean (SD) | −22.1 (7.0) | −20.9 (7.8) | −14.9 (6.3) | −23.7 (7.3) | 0.001 |
| Mid Anteroseptal Strain | |||||
| N (N missing) | 35 (0) | 35 (0) | 28 (7) | 34 (1) | |
| Mean (SD) | −17.8 (4.8) | −19.0 (6.2) | −15.4 (6.1) | −19.1 (4.8) | 0.05 |
| Mid Posterior Strain | |||||
| N (N missing) | 30 (5) | 25 (10) | 26 (9) | 27 (8) | |
| Mean (SD) | −21.1 (6.5) | −17.2 (8.9) | −20.2 (8.8) | −18.8 (8.8) | 0.07 |
| 4 Chamber | |||||
| Apical Lateral Strain | |||||
| N (N missing) | 33 (2) | 34 (1) | 32 (3) | 35 (0) | |
| Mean (SD) | −21.5 (8.2) | −21.4 (10.7) | −19.7 (5.1) | −22.3 (9.0) | 0.18 |
| Apical Septal Strain | |||||
| N (N missing) | 35 (0) | 35 (0) | 31 (4) | 35 (0) | |
| Mean (SD) | −15.6 (7.7) | −16.4 (7.2) | −24.8 (6.3) | −18.2 (6.7) | <.0001 |
| Basal Lateral Strain | |||||
| N (N missing) | 34 (1) | 33 (2) | 32 (3) | 34 (1) | |
| Mean (SD) | −18.4 (7.5) | −20.7 (8.2) | −14.7 (5.2) | −20.0 (7.9) | <.0001 |
| Basal Septal Strain | |||||
| N (N missing) | 35 (0) | 35 (0) | 32 (3) | 35 (0) | |
| Mean (SD) | −15.2 (3.2) | −16.8 (5.8) | −14.9 (3.8) | −16.7 (6.0) | 0.15 |
| Mid Lateral Strain | |||||
| N (N missing) | 34 (1) | 34 (1) | 32 (3) | 33 (2) | |
| Mean (SD) | −22.1 (6.7) | −19.7 (7.5) | −21.9 (5.2) | −19.1 (9.0) | 0.10 |
| Mid Septal Strain | |||||
| N (N missing) | 35 (0) | 35 (0) | 32 (3) | 35 (0) | |
| Mean (SD) | −16.1 (4.0) | −18.1 (4.1) | −15.3 (4.0) | −19.6 (5.5) | 0.001 |
Assessment of differences across scanners
Strain measurements between images from different scanners and their VIS output demonstrated considerable variability in regional strain measurements compared to global strain measurements as assessed by their coefficient of variation (CoV), with CoV in the range of 25–35% for regional strain measures compared to CoV of 10–15% for global strain measures (Supplementary Table 2). There was no significant difference between global strains obtained from images from different scanners. The only statistically significant difference in regional strains was in apical inferior strain in the 2-chamber view where on average Philips was ~3.3% more negative than GE (p=0.02). Otherwise, no statistically significant differences were noted, likely due to the high variability in regional strains.
When comparing VSS, there was numerically higher differences between both global (2-chamber) strain and multiple regional strain values in different views (Supplemental Table 3). Although there were some statistically significant differences between VSS strains, no consistent direction to the differences was noted. Overall, there were fewer significant systematic differences between strains from VIS derived from different vendor images compared to strains from VSS derived from those same images, although the magnitudes of the between-replicate variability (as assessed by CoV) were generally similar regardless of whether VIS or VSS was used.
Assessment of differences across software and software
Strain derived from images from both scanners (Supplemental Tables 4 and 5) VSS were individually compared with VIS (Tomtec, both Average and LoL for global strains). Global GE strain had statistically significant differences in the 2-chamber and 4-chamber views compared to the VIS-LoL measurements, which yielded higher (more negative) strain. The Philips system had a statistically significant difference in the 4-chamber LoL results with Tomtec again showing higher strain, but no other differences were noted. Both VSS regional strains demonstrated substantial variability that included several statistically significant values without any clear systematic trend in direction.
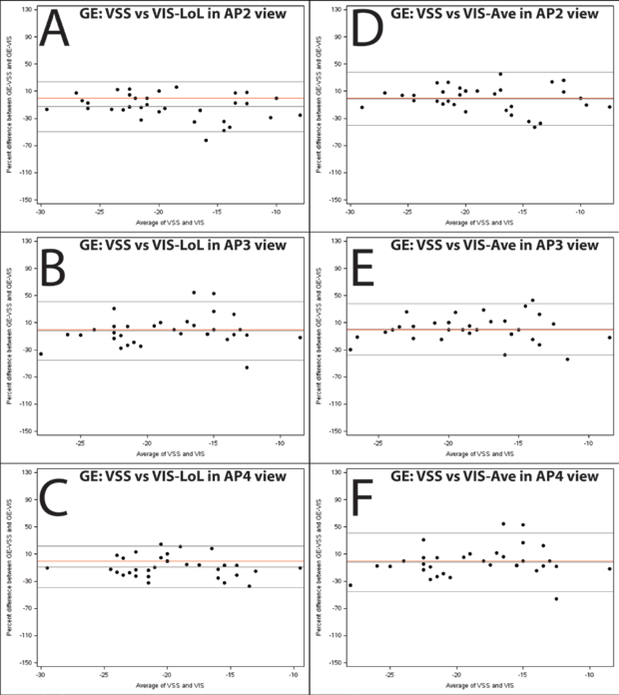
Bland-Altman plots of agreement were created for the global RVLS of each of the different chamber views for comparison (Figures 3–6). For example, Figure 5A–C illustrate the slight systematic bias between GE VSS and TomTec LoL measures of global strain. This helps to illustrate the magnitude of the systematic difference relative to the variability (how wide apart the Limit of Agreement lines are). No major systematic differences between scanner/software combinations were found, although there was noticeable variability in the data.
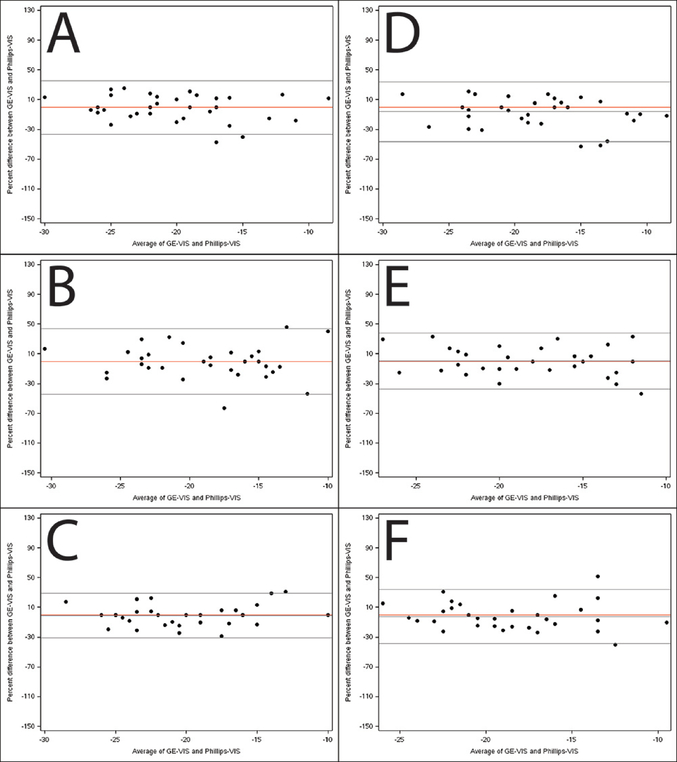
Figure 3. Bland-Altman plots illustrating agreement between global strain measured by vendor-independent software (TomTec) on images acquired by the GE and Philips scanners.
Percentage difference is the difference between GE-VIS and Philips-VIS divided by the mean of the paired measurements. Panels A-C depict agreement between the TomTec length of line global strain measurements (2, 3, and 4 chamber respectively) and panels D-F depict agreement between the TomTec average global strain measurements. The middle black line depicts the average % difference and the outer lines depict the 95% limits of agreement. The red line is a reference for zero bias.
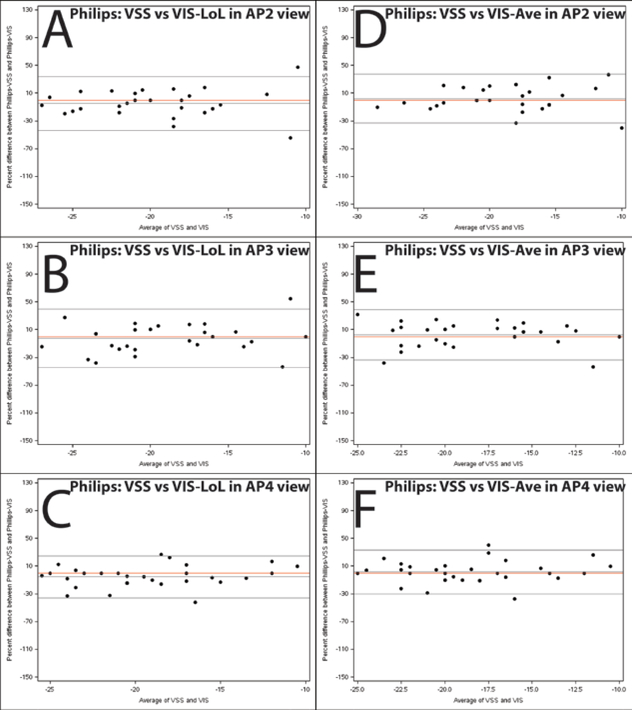
Figure 6. Bland-Altman plots illustrating agreement between global strain measured by VSS and VIS on images acquired using the Philips iE33 system.
Percentage difference is the difference between Philips-VSS and Philips-VIS divided by the mean of the paired measurements. Panels A-C depict agreement between the Philips software global strain measurement and the TomTec Length of Line global strain measurement for 2, 3, and 4 chamber respectively. Panels D-F depict agreement between the Philips software global strain measurement and the TomTec average global strain measurement. The middle black line depicts the average % difference and the outer lines depict the 95% limits of agreement. The red line is a reference for zero bias.
Figure 5. Bland-Altman plots illustrating agreement between global strain measured by VSS (GE EchoPac) and VIS (TomTec 2D cardiac Performance Analysis) on images acquired using the GE Vivid 9 system.
Percentage difference is the difference between GE-VSS and GE-VIS divided by the mean of the paired measurements. Panels A-C depict agreement between the GE software global strain measurement and the TomTec Length of Line global strain measurement for 2, 3, and 4 chamber respectively. Panels D-F depict agreement between the GE software global strain measurement and the TomTec average global strain measurement. The middle black line depicts the average % difference and the outer lines depict the 95% limits of agreement. The red line is a reference for zero bias.
Discussion
This study sought to investigate the reproducibility of various RV global and regional strain analyses between different ultrasound vendors using VSS, as well as to evaluate the agreement between RV strain measurements obtained using VIS. Overall, wide variability was found, particularly for regional strain measurements, but without any clearly identifiable systematic influences. The only exception to this was for several global views that showed higher (more negative by about 2% on average) strain measurements using VIS LoL software compared to other methods. The higher LoL strain and global strain from VIS could be due to differences in reporting subendocardial strain rather than average full myocardial thickness or inclusion of non-myocardium from VSS. This may also explain some of the variability in regional strains as well. Overall, the small degree of bias we observed between VSS and VIS is not likely to be clinically relevant. The significant variability, with a general lack of bias, would preclude the use of any hypothetical correction between VSS and VIS regional strains. Our data on reproducibility support the use of RV global strain but do not support the use of RV regional strains across vendor platforms, due to the significant variability seen in regional, but not global strains.
In a study of similar design focusing on LV strain,16 it was found there was good reproducibility for global longitudinal strain, but only moderate reproducibility for circumferential strain, and poor reproducibility for radial strain. As 70% of the contractile function of the RV is longitudinal19, it is reasonable to posit that RVLS would demonstrate good reproducibility across different ultrasound platforms and software packages. The question of reproducibility is important for several reasons. First, the widespread use of RVLS could be hindered by the uncertainty of different vendor packages introducing systematic error because of differences in the proprietary algorithms used to calculate strain. While this question has been addressed for the LV16, 17, until this study, those results could not be extrapolated to the RV because of its complex anatomy and the lack of readily available RV specific strain analysis software. Second, the ability to confidently compare data across studies from different machines and centers is advantageous from both a clinical and research perspective.
Strain measurements from 2DSTE can evaluate both global and regional ventricular function, and strain technology applied to the LV has demonstrated to be a more sensitive measure of LV function than LV ejection fraction (LVEF). For example, in patients treated with anthracycline chemotherapy, changes in LV longitudinal strain were found to precede subsequent decreases in LVEF.22 This suggests that RVLS could also be more sensitive to changes in RV function than RVEF. In conditions in which RV function is closely tied to outcomes (like PH), RVLS may be the most effective way to monitor RV function in these patients.23
Recent clinical trials in PH have tended not to use echo as an endpoint24, primarily due to the lack of validated echo measures of RV function. Validated reproducibility for RVLS will be important for any multicenter trial that uses RVLS as a surrogate marker. In clinical practice, this could allow RVLS to be used to monitor patients with PH, congenital heart disease, pulmonary embolism and other disease in which RV function is known to be associated with outcomes.
Limitations
This study had several limitations. First, while there are multiple ultrasound platforms available, this study only compared two to a single VIS. Second, scans on the same patient were not repeated with the same machine, which would allow an assessment of variability from scan to scan. Third, the strain algorithms used in VSS and VIS in this study have been validated for LV strain and not designed for assessing RV strain. Despite this limitation, algorithms to determine strain are not chamber specific. Fourth, the range and interpretation of what constitutes reasonable agreement and reproducibility can be subjective as there are no established cut-offs for such studies. Fifth, the study population tested here was fairly small, though comparable to a previous similar study of the left ventricle.16 Lastly, it is recognized that ultrasonic speckle from different imaging systems are different in size and position and depend on interrogating frequency, sampling rate and other factors. As well, GE and Philips track speckle from raw data (pre DICOM) with differing algorithms, but with different definitions of the mid myocardium. TomTec determines strain from the endocardium from DICOM spatially and temporally compressed images. GE and Philips do not allow for strain determination on compressed DICOM images and TomTec is disallowed from determining strain from manufacturers raw data. There are no readily available strain phantoms as there are for dimensional or volumetric determination. These analytic programs are, obviously, not all working on the same substrate ultrasonic speckle information or methodologies, but comparisons are, nevertheless, necessary in order to use strain in any clinical context.
Conclusions
A comparison of strains measured between vendor-specific and vendor-independent imaging analyses demonstrated that, despite widespread variability in the data, there were no major systematic differences with vendor-specific imaging or approaches for calculating RVLS, despite system differences. There was acceptable variability for global RVLS but large variability (sometimes 30–40%) for regional strains, which make regional RVLS less appealing. These findings suggest that global RVLS could continue to serve as an important emerging method to characterize RV function and has the required reproducibility for future clinical and research applications.
Supplementary Material
Figure 2. Global strains from VSS and VIS for images obtained from different scanners.
VIS-Ave: VIS average strain; VIS-LoL: VIS length-of-line strain. Shown are strains (−%) with standard deviation.
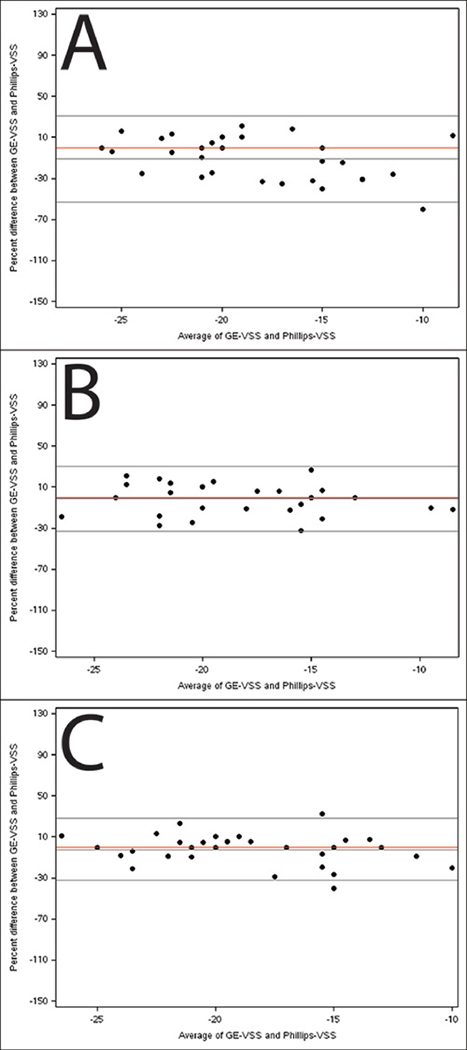
Figure 4. Bland-Altman plots illustrating agreement between global strain measured by vendor-specific software on images acquired by the GE and Philips scanners.
Percentage difference is the difference between GE-VSS and Philips-VSS divided by the mean of the paired measurements. Panels A-C depict agreement between the GE software global strain measurements and the Philips software global strain measurements for 2, 3, and 4 chamber respectively. The middle black line depicts the average % difference and the outer lines depict the 95% limits of agreement. The red line is a reference for zero bias.
Acknowledgments
Disclosures
This study was funded as an investigator-initiated study to SR and ZS from Gilead Sciences.
JK is a speaker for Philips and GE and holds grants from Medtronic, Philips and Novaseed.
SR: Research support from NIH K08HL114643, Burroughs Wellcome Career Award for Medical Scientists, Actelion and United Therapeutics; Consultancy/Honorarium from Actelion, United Therapeutics and Gilead Sciences.
EJV: Research support from NHLBI, Novartis, Amgen, Pfizer, Alnylam, Bay Labs, Philips, and GE; Consultancy/Honorarium from Novartis, Amgen, Philips, Merck, Abiomed and Expert Exchange
EJV is supported by R01 HL105853, R01 HL118077, U10 HL084904 and U01 HL125511
ZJI, HM, KC, KA, JT, and AH have no disclosures
References
- 1.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 2.Forsha D, Risum N, Kropf PA, Rajagopal S, Smith PB, Kanter RJ, et al. Right ventricular mechanics using a novel comprehensive three-view echocardiographic strain analysis in a normal population. J Am Soc Echocardiogr. 2014;27:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–309. [DOI] [PubMed] [Google Scholar]
- 4.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 5.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:711–21. [DOI] [PubMed] [Google Scholar]
- 6.Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:628–36. [DOI] [PubMed] [Google Scholar]
- 7.Giusca S, Jurcut R, Coman IM, Ghiorghiu I, Catrina D, Popescu BA, et al. Right ventricular function predicts clinical response to specific vasodilator therapy in patients with pulmonary hypertension. Echocardiography. 2013;30:17–26. [DOI] [PubMed] [Google Scholar]
- 8.Ladouceur M, Redheuil A, Soulat G, Delclaux C, Azizi M, Patel M, et al. Longitudinal strain of systemic right ventricle correlates with exercise capacity in adult with transposition of the great arteries after atrial switch. Int J Cardiol. 2016;217:28–34. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury SM, Hijazi ZM, Fahey JT, Rhodes JF, Kar S, Makkar R, et al. Speckle-Tracking Echocardiographic Measures of Right Ventricular Function Correlate With Improvement in Exercise Function After Percutaneous Pulmonary Valve Implantation. J Am Soc Echocardiogr. 2015;28:1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassoun PM, Nikkho S, Rosenzweig EB, Moreschi G, Lawrence J, Teeter J, et al. Updating clinical endpoint definitions. Pulm Circ. 2013;3:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradlow WM, Gibbs JS, Mohiaddin RH. Cardiovascular magnetic resonance in pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopal S, Forsha DE, Risum N, Hornik CP, Poms AD, Fortin TA, et al. Comprehensive assessment of right ventricular function in patients with pulmonary hypertension with global longitudinal peak systolic strain derived from multiple right ventricular views. J Am Soc Echocardiogr. 2014;27:657–65 e3. [DOI] [PubMed] [Google Scholar]
- 13.Forsha D, Risum N, Rajagopal S, Dolgner S, Hornik C, Barnhart H, et al. The influence of angle of insonation and target depth on speckle-tracking strain. J Am Soc Echocardiogr. 2015;28:580–6. [DOI] [PubMed] [Google Scholar]
- 14.Dahhan T, Siddiqui I, Tapson VF, Velazquez EJ, Sun S, Davenport CA, et al. Clinical and echocardiographic predictors of mortality in acute pulmonary embolism. Cardiovasc Ultrasound. 2016;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–93. [DOI] [PubMed] [Google Scholar]
- 16.Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–203. [DOI] [PubMed] [Google Scholar]
- 17.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28:1171–81, e2. [DOI] [PubMed] [Google Scholar]
- 18.Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K, et al. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr. 2015;28:630–41. [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48. [DOI] [PubMed] [Google Scholar]
- 20.Forsha DE, Risum N, Smith PB, Kropf A, Rajagopal S, Samad Z, et al. A Novel Comprehensive RV Strain Analysis: Echocardiographic Approach to Define a Normal Adult Population. J Am Soc Echocardiogr. 2014:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–9. [DOI] [PubMed] [Google Scholar]
- 22.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–40. [DOI] [PubMed] [Google Scholar]
- 23.Reichek N. Right ventricular strain in pulmonary hypertension: flavor du jour or enduring prognostic index? Circ Cardiovasc Imaging. 2013;6:609–11. [DOI] [PubMed] [Google Scholar]
- 24.Parikh KS, Rajagopal S, Arges K, Ahmad T, Sivak J, Kaul P, et al. Use of outcome measures in pulmonary hypertension clinical trials. Am Heart J. 2015;170:419–29 e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.