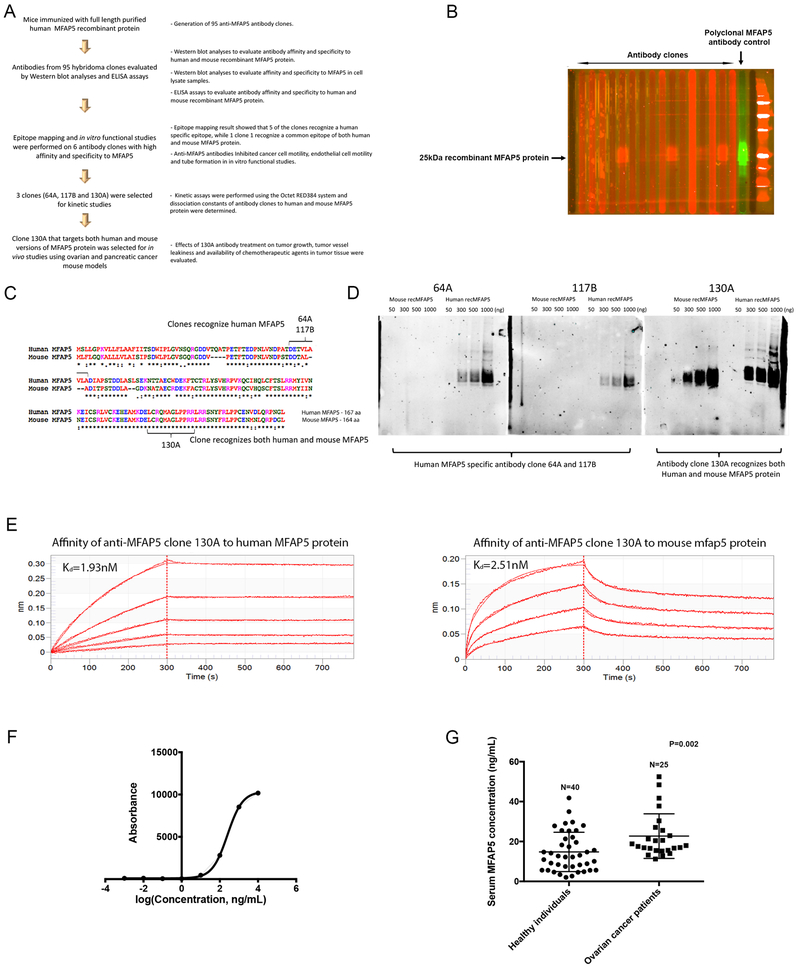
Figure 1. Development and characterization of anti-MFAP5 monoclonal antibodies.
(A) A flow diagram illustrating the overall workflow of therapeutic anti-MFAP5 monoclonal antibody development. (B) Western blot analyses were performed to identify antibody clones with high binding affinity and specificity to MFAP5. (C) Epitope mapping results showing that whereas clones 64A and 117B recognized human MFAP5, clone 130A recognized both human and murine MFAP5 protein sequences. (D) Western blot analyses validating the specificity of two clones of mouse monoclonal antibodies against human MFAP5 (64A and 117B) and one clone of an antibody against both human and mouse MFAP5 (130A). (E) Kinetic assay results showed that the dissociation constant (Kd) of clone 130A for human and mouse MFAP5 protein were at 1.93nM and 2.51nM respectively, suggesting that clone 130A has high binding affinity to both human MFAP5 and mouse Mfap5 protein (F) A titration curve for the 130A antibody generated by ELISA and recombinant MFAP5 protein at various concentrations. (G) ELISA results demonstrating that serum samples obtained from high grade serous ovarian cancer patients had significantly higher levels of MFAP5 than the healthy individuals did.