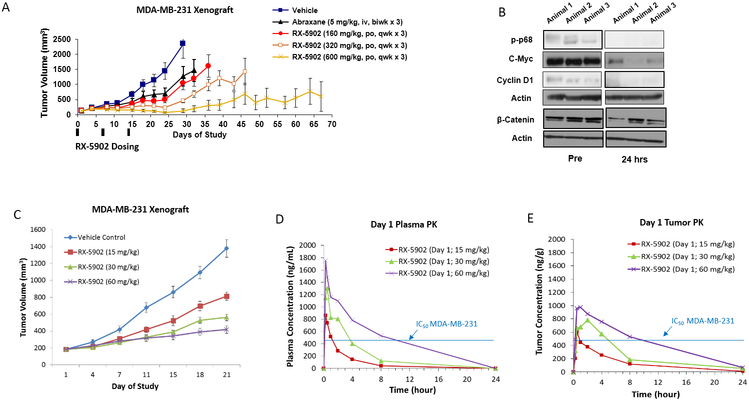
Figure 4: RX-5902 has anti-tumor activity against a MDA-MB-231 xenograft model, dose-proportional pharmacokinetics and treatment results in decreased phosphorylated-p68, c-myc and β-catenin.
(A) Effect of RX-5902 administered once weekly in the MDA-MB-231 xenograft model. Animals were randomized to treatment with vehicle, nab-paclitaxel 5 mg/kg IV twice a week × 3, RX-5902 160 mg/kg via oral gavage once weekly × 3, RX-5902 320 mg/kg once weekly × 3 or RX-5902 600 mg/kg once weekly × 3. Nab-paclitaxel was used as a chemotherapy control. Tumor growth inhibition (TGI) RX-5902 160 mg/kg 55.7%, 320 mg/kg 80.29% and 600 mg/kg 94.58%, p < 0.001. TGI nab-paclitaxel 45%. B) Immunoblots performed using protein isolated from tumor tissue obtained pre and 24h post RX-5902. Three mice were included in each group. Immunoblotting was performed for p-p68, C-myc, Cyclin D1, β-catenin and actin. C) Based on observed toxicity in the ongoing Phase I dose escalation study, the effect of lower dose RX-5902 5 days on 2 days off was investigated in the MDA-MB-231 xenograft model. TGI RX-5902 15 mg/kg 45%, 30 mg/kg 58% and 60 mg/kg 72%, p < 0.001. D) Plasma concentration-time curve after a single dose of RX-5902 15 mg/kg, 30 mg/kg or 60 mg/kg in thenMDA-MB-231 xenograft model in nude mice. E) Tumor concentration-time curve after a single dose of RX-5902 15 mg/kg, 30 mg/kg or 60 mg/kg in the MDA-MB-231 xenograft model in nude mice.