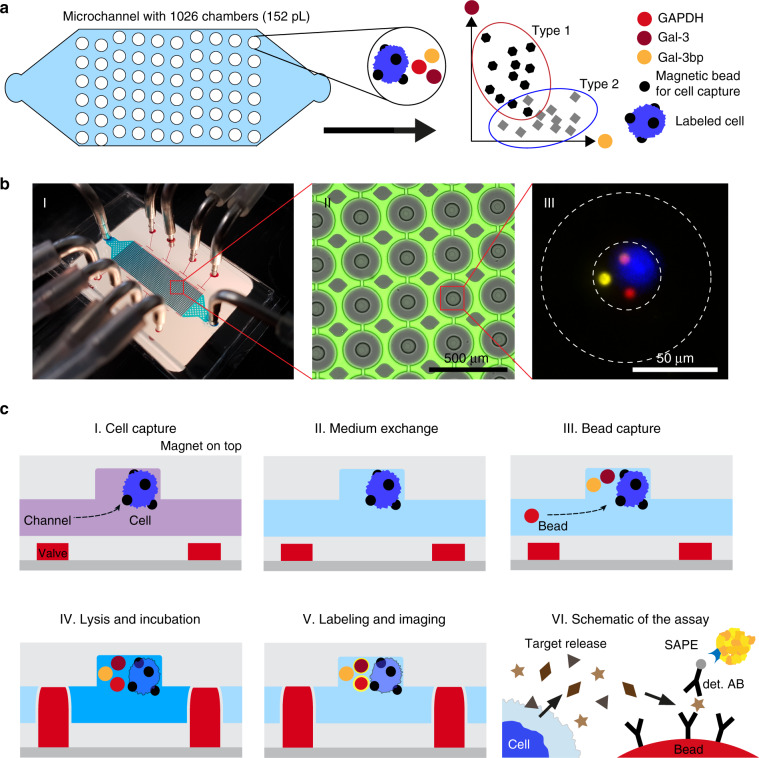
Fig. 1. Microfluidic chip design and functionality for single-cell protein profiling.
a Schematic of the microfluidic chip with 1026 individual microfluidic chambers used for single-cell analysis. Protein quantification was performed by immunoassays with fluorescently barcoded beads, which were co-immobilized with the cells of interest. b Images of the chip and microchambers. (I) Photograph of the chip with the tubing used for the supply of the liquids (blue) as well as the eight control lines (red) used to activate the valves. The permanent magnet that is positioned on top of the chip during the entire experimental procedure is not shown. (II) Micrograph of the chamber array depicting the actuated round valves in grey, as well as the surrounding fluorescent solution (green). The columns between the microchambers (in grey) were used to stabilize the microchannel. (III) A composite fluorescent image of one chamber holding a single cell (blue colour) and three barcoded beads (yellow, red and pink colour) for multiplexed immunoassays. c The protocol used for sensitive proteomic profiling included (I) cell capture, (II) medium exchange, (III) co-capture of beads, (IV) cell lysis and incubation, (V) fluorescent labelling and final imaging. (VI) The immunoassays with the beads required a two-step labelling procedure, first with the biotinylated detection antibodies (det. AB) and subsequently with streptavidin-PE (SAPE)