Abstract
Background:
Abdominal exploration followed by vascular bypass has been the standard of care for acute mesenteric ischemia (AMI), but there is increasing use of endovascular treatment with selective exploratory laparotomy.
Methods:
We performed a retrospective review of patients diagnosed with AMI who underwent mesenteric artery angioplasty or stenting at a single institution from 2010-2017. Patients were divided into 3 groups: those who did not undergo exploratory laparotomy; those who received endovascular treatment before laparotomy (post-reperfusion laparotomy group); and those who had endovascular treatment after laparotomy (pre-reperfusion laparotomy group).
Results:
Patients who did not undergo exploratory laparotomy showed 85.7% (12/14) survival, compared with 63.6% (7/11) in the post-reperfusion group and 25.0% (2/8) in the pre-reperfusion group, P=0.077). Time to reperfusion was significant (P=0.009) in predicting survival for patients who underwent exploratory laparotomy.
Conclusion:
Emergent endovascular treatment prior to laparotomy seems to be associated with a higher survival.
Keywords: Acute mesenteric ischemia, endovascular therapy, stenting
Introduction
Acute mesenteric ischemia (AMI) is a rare (<1 in every 1000 hospital admissions) but deadly syndrome in which there is a sudden reduction in the blood supply to the intestine, leading to ischemia and possible bowel infarction [1]. The 4 major types of AMI are: acute superior mesenteric artery thromboembolic occlusion (40-50%); and non-occlusive mesenteric ischemia (20-30%); mesenteric arterial thrombosis (25%); mesenteric venous thrombosis (<5%) [2]. Other etiologies include traumatic injury, vasculitis, dissection of the aorta, intestinal obstruction, and cholesterol emboli. The overall mortality rate of AMI is 50-70% and reaches 90% in the case of bowel infarction [2]. Peripheral occlusions are associated with lower mortality rates than central occlusions, and non-occlusive AMI yields an even poorer prognosis because of its uncharacteristic presentation, which can lead to delayed diagnosis and irreversible damage, and because it tends to occur in sicker populations. In proximal occlusive arterial disease, patient survival is contingent on revascularization before ischemia progresses to intestinal gangrene [1].
AMI is difficult to diagnose based on clinical presentation alone, because of its similarities to various abdominal pathological conditions. There are no serum markers specific or sensitive to diagnose AMI alone, and, although higher serum lactate levels are associated with increased AMI mortality, normal serum lactate levels do not rule AMI out [3]. Furthermore, AMI is an uncommon cause of emergency room visits, which results in a lack of clinical suspicion and delayed presentation. The diagnostic test of choice for occlusive AMI is computed tomography (CT) with arterial and portal venous phase contrast administration, along with 3D multiplanar reconstruction (MPR)-CT, which allows for the evaluation of secondary signs of AMI as well as an assessment of detailed vascular anatomy [1].
Endovascular techniques, hybrid techniques or conventional surgery can be used for reperfusion. The choice of endovascular reperfusion is often guided by whether signs of acute bowel infarction are present. Depending on the patient’s clinical status, endovascular reperfusion may be considered in the setting of acute bowel infarction if reperfusion can be re-established in an expeditious fashion. However, some authors have suggested that percutaneous catheter-based techniques using transfemoral or transaxillary access could be favored over open vascular reconstruction, given the high comorbidity of the patient population [4]. Because endovascular procedures are less invasive, they are believed to be associated with reduced mortality, and several studies have demonstrated increased survival rates among AMI patients who underwent endovascular therapy [4-6]. However, the overall mortality of AMI has remained constant in the past decade despite the increased utilization of endovascular therapy [7]. The objective of this study was to review the management and outcome of patients with AMI who had undergone angioplasty or stent procedures.
Patients and methods
An institutional review board-approved clinical repository at a single institution was searched for patients with a discharge diagnosis of AMI and produced 1083 results. The clinical records of all patients with this diagnosis between 2010 and 2017 were retrospectively reviewed. From this list, the criteria for AMI were defined as acute onset of severe abdominal pain, with or without peritoneal signs on examination, but without other sources of abdominal pain. Diagnosis of AMI was confirmed by either laboratory measures of bowel injury (lactic acid >2.2 mg/dL), imaging (plain film, CT, angiography), endoscopy or surgical exploration. Of those with severe AMI, 33 patients underwent angioplasty and/or stenting of one or more of the mesenteric arteries with or without thrombectomy or thrombolysis.
The demographic information, body mass index (BMI), vital signs, comorbid conditions, presenting symptoms, AMI risk factors, surgical history, imaging results, procedures, time between admission and reperfusion, and patient outcomes were extracted by chart review. Specific outcomes of interest were post-procedural complications, number of revisits to the operating room, development of morbidities, bowel surgery and mortality rates. Technical success of endovascular treatment was defined as the ability to cross a lesion, provide proximal patency with no stenosis >30%, and improve intestinal perfusion according to subjective angiographic evaluation at the time of the procedure. Post-procedure complications were defined as mesenteric artery dissection, stent dislodgement, distal embolization, thrombosis, or perforation that resulted directly from the procedure [8]. Of the patients who survived, follow-up endpoints observed were the ability to take food in orally, BMI, imaging results from CT or angiography, and the need for re-intervention. Time to reperfusion, 30-day survival percentage and lactic acid levels on presentation were compared between the 3 groups of patients: no exploratory laparotomy, post-perfusion exploratory laparotomy, and pre-reperfusion exploratory laparotomy. In general, patients with clinical signs of peritonitis, hemodynamic instability, or signs of perforation on scans received a laparotomy first.
Statistical analysis
Microsoft Excel was used for all statistical analyses. The 2-tailed t-test was used to compare differences in continuous variables between patients who survived and patients who expired. Chi-square test was used to compare 30-day survival percentages between the 3 groups of patients. One-way analysis of variance was used to calculate the significance of differences in survival between groups with continuous variables (lactic acid levels and time to reperfusion). Charlson comorbidity index (CCI) was also calculated for each patient to compare baseline survival.
Results
The mean age was 65±12.2 years and mean BMI was 25.8±6.23 kg/m2. Of the 33 patients, 60.6% were women, 78.8% were smokers, and 72.7% were taking anticoagulant or antiplatelet medication on admission. The most common comorbid conditions were all related to cardiovascular disease and included hypertension (84.9%), hyperlipidemia (63.3%), coronary artery disease (51.5%), peripheral vascular disease (48.5%), and diabetes mellitus (39.4%) (Table 1). Thirty-six percent of the patients had a history of chronic mesenteric ischemia and 48% had a prior CT scan that showed evidence of mesenteric vessel stenosis. The presenting symptoms of these patients were, in order of decreasing frequency, abdominal pain and tenderness (96.9% and 84.8%), nausea and vomiting (60.6%), and diarrhea (45.4%). The average CCI was 4.30.
Table 1.
Demographics of patients with acute mesenteric ischemia undergoing mesenteric artery angioplasty
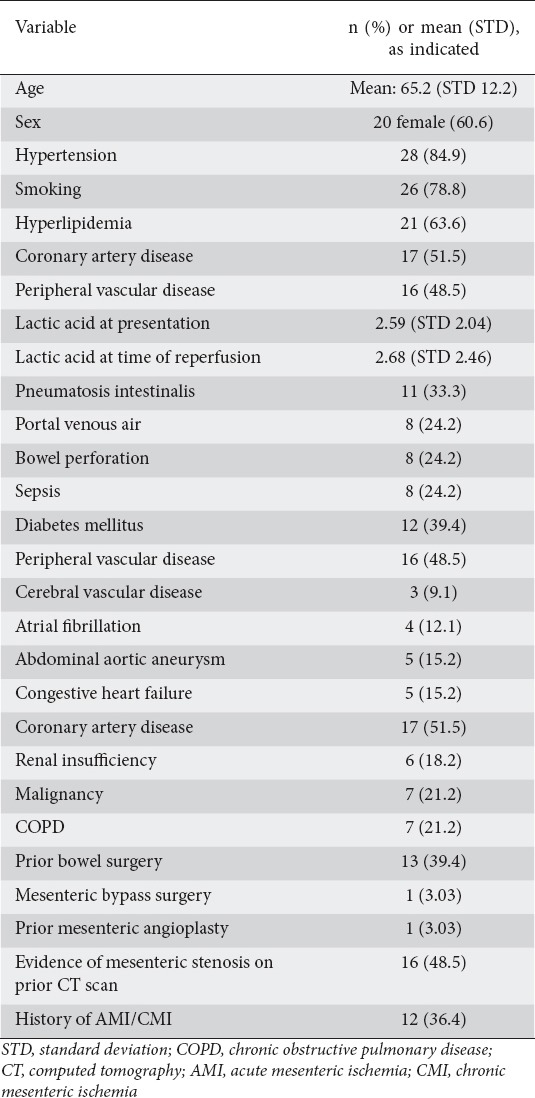
Technical success was high at 100% (33/33), similar to previously reported data [9]. Stents were placed in 28/33. The remaining 5 patients who did not undergo stent placement were treated with angioplasty of an existing stent, embolectomy and/or nitroglycerin/papaverine therapy. Procedure-related complications were seen in 18% of patients (6/33); all these 6 patients had AMI due to thrombosis.
White blood cell counts were elevated (15.58±6.2 × 109/L), as were lactic acid levels on admission (2.59±2.04 mmol/L) and before reperfusion (2.68±2.46 mmol/L) (Table 2). Patients who survived had a significantly lower lactic acid level on presentation compared with patients who expired (2.19 vs. 3.82 mmol/L; P=0.0344), as well as a significantly lower lactic acid level before reperfusion (1.74 vs. 4.18 mmol/L; P=0.015). As for imaging data, the most common vessel occluded or narrowed was the superior mesenteric artery. Of the 33 patients, 60% underwent exploratory laparotomy (23.3% before reperfusion, 36.7% after). The median number of narrowed vessels seen on imaging was 2.
Table 2.
Variables predicting survival of acute mesenteric patients undergoing angioplasty
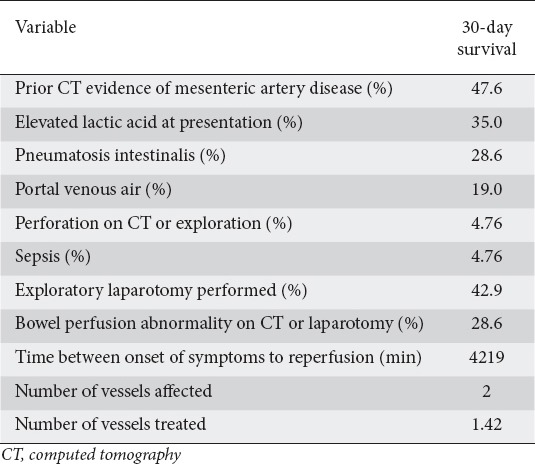
While the total time from onset of AMI symptoms to reperfusion did not differ significantly between the patients who survived and those who did not (P=0.39), we saw significant differences in reperfusion times for patients who underwent exploratory laparotomy (pre-reperfusion 8475 min vs. post-reperfusion 3049 min; P=0.009). We also saw differences in 30-day survival percentage (pre-reperfusion 25% vs. post-reperfusion 60%; P=0.077), but results were statistically insignificant.
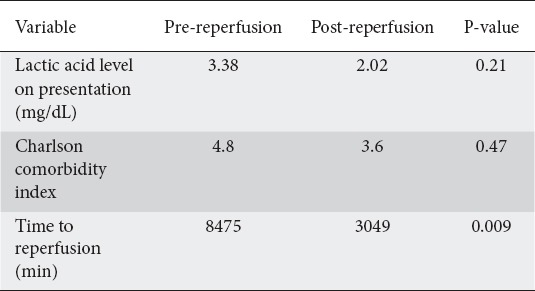
The CCI did not differ significantly between the patients in the pre-reperfusion and post-reperfusion groups (4.8 vs. 3.6; P=0.47). There were also no significant differences in lactic acid levels between the pre-reperfusion and post-reperfusion exploratory laparotomy groups (mean 3.38 and 2.02 mg/dL; P=0.21) (Table 3).
Table 3.
Comparison between patients who received exploratory laparotomy
Outcomes were also significantly different between the 3 groups. The pre-reperfusion group had a median of 2.5 revisits, the post-reperfusion group a median of 1 revisit, and the no exploratory laparotomy group a median of 0 revisits. A significantly higher proportion of patients from the pre-reperfusion exploratory laparotomy group progressed to sepsis when compared to the 2 other groups (0.86 vs. 0.13, P=0.0005).
Other factors that correlated with 30-day mortality included the development of sepsis, having more than 2 revisits to the operating room, and having a higher lactic acid level upon admission and before reperfusion. Many of these factors varied significantly in the 3 laparotomy groups, which contributed to their differences in 30-day survival percentage.
Discussion
Our study showed that 30-day survival varied tremendously amongst patients with AMI. In those managed with endovascular reperfusion, particularly those who could avoid exploratory laparotomy, survival appears to be higher than that reported in the existing literature (85.7% vs. 50-70%) [2]. Patients who underwent exploratory laparotomy before reperfusion had 25% 30-day survival, much lower compared with previous publications [2].
There was no significant difference overall in times to reperfusion between patients who survived and patients who did not. A likely explanation for this finding is that patients who show up in a more critical condition often receive more emergent treatment, thus shortening their time to reperfusion. Patients not critical and more likely to survive tend to have to wait longer for further confirmation or signs of AMI. When we further divided the exploratory laparotomy patients into 2 groups, endovascular intervention before or after reperfusion, we saw significant differences in both 30-day survival and reperfusion times: a shorter time to reperfusion was associated with a higher survival percentage. Branco et al showed that “endovascular therapy first” was associated with a decrease in risk of death compared to traditional laparotomy as first approach (odds ratio 0.4, 95% confidence interval 0.2-0.9; P=0.018) [10]. Dhamnaskar et al also demonstrated that time was a significant factor in the survival of patients with AMI [10]. In their study, the mortality rate was significantly higher in patients who presented late. Time to diagnosis also played a role; survival rate dropped from 50% to 30% after the first 24 h [11].
The need for an exploratory laparotomy seems to be associated with a more severe presentation of AMI. For that group of patients, percutaneous interventions provide the quickest way to reperfusion despite distal embolization. The data suggest that time to reperfusion in patients who require exploratory laparotomy plays a role in increased 30-day survival. The difference might also be explained by the severity of symptoms, imaging findings or bowel injury. However, there were no significant differences in comorbid conditions, including lactic acid levels, between the pre-reperfusion and post-reperfusion laparotomy groups. Prior studies have also suggested that endovascular therapies are associated with better survival compared to traditional laparotomies [4-6]. A retrospective cohort review performed in 2011 showed that successful endovascular therapies resulted in a mortality rate of 36%, compared to 50% with traditional therapy [4]. A larger study in 2014 that compared endovascular and traditional therapy showed that, despite similar CCIs, endovascular therapy had a mortality of 24.9% compared to 39.3% for open interventions [6]. Endovascular revascularization also resulted in shorter hospital stays compared with open revascularization (12.9 vs. 17.1 days; P=0.006) and a lower requirement for total parenteral nutritional support (13.7% vs. 24.4%; P=0.025) [6].
With only 33 subjects, the study is limited by its small population. Like every retrospective chart review, this study may suffer from selection bias and inability to show causation. Additionally, not all patient charts had the same degree of chart detail and not all patients had the same laboratory tests. Liver enzymes, for example, were only tested in 2/3 of the patients. Including randomization in the treatment algorithm will be difficult, but a larger prospective study with better control of other risk factors may be possible. Evaluation of imaging is also subjective. For example, differentiating embolus from spasm can be difficult. We also recognize that local practice dictates that more critical patients must be taken for exploratory laparotomy first. We acknowledge this selection bias and recognize how it may affect our data analysis.
This study suggests that the timing of exploratory laparotomy, before or after endovascular treatment, plays an important role in predicting the survival of more critical AMI patients. Both the severity of symptoms and the CT results make immediate reperfusion in this group more vital than ever. The findings from this study may have future implications for the management of AMI, and the timing of exploratory laparotomy and time to reperfusion may be important factors in striving for improved survival. In select populations in which endovascular therapy is favorable, exploratory laparotomy should be performed after reperfusion, but further research is needed into patient selection in order to determine the optimal timing of exploratory laparotomies and endovascular intervention.
Summary Box.
What is already known:
Acute mesenteric ischemia has a high mortality rate and remains difficult to diagnose
Endovascular intervention has a lower mortality rate compared to traditional open treatment
The technical success rate of endovascular intervention is high
What the new findings are:
The need for exploratory laparotomies was associated with higher mortality
For patients who needed exploratory laparotomies, the time to reperfusion was associated with mortality
Patients who needed exploratory laparotomy first had poorer outcomes
Biography
University of Virginia School of Medicine, USA
Footnotes
Conflict of Interest: None
References
- 1.Wyers MC. Acute mesenteric ischemia:diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9–20. doi: 10.1053/j.semvascsurg.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Stone JR, Wilkins LR. Acute mesenteric ischemia. Tech Vasc Interv Radiol. 2015;18:24–30. doi: 10.1053/j.tvir.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg. 2007;246:323–329. doi: 10.1097/SLA.0b013e3180603010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthurs ZM, Titus J, Bannazadeh M, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698–704. doi: 10.1016/j.jvs.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Schermerhorn ML, Giles KA, Hamdan AD, Wyers MC, Pomposelli FB. Mesenteric revascularization:management and outcomes in the United States, 1988-2006. J Vasc Surg. 2009;50:341–348. doi: 10.1016/j.jvs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaulieu RJ, Arnaoutakis KD, Abularrage CJ, Efron DT, Schneider E, Black JH., 3rd Comparison of open and endovascular treatment of acute mesenteric ischemia. J Vasc Surg. 2014;59:159–164. doi: 10.1016/j.jvs.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 7.Eslami MH, Rybin D, Doros G, McPhee JT, Farber A. Mortality of acute mesenteric ischemia remains unchanged despite significant increase in utilization of endovascular techniques. Vascular. 2016;24:44–52. doi: 10.1177/1708538115577730. [DOI] [PubMed] [Google Scholar]
- 8.Oderich GS, Tallarita T, Gloviczki P, et al. Mesenteric artery complications during angioplasty and stent placement for atherosclerotic chronic mesenteric ischemia. J Vasc Surg. 2012;55:1063–1071. doi: 10.1016/j.jvs.2011.10.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ierardi AM, Tsetis D, Sbaraini S, et al. The role of endovascular therapy in acute mesenteric ischemia. Ann Gastroenterol. 2017;30:526–533. doi: 10.20524/aog.2017.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branco BC, Montero-Baker MF, Aziz H, Taylor Z, Mills JL. Endovascular therapy for acute mesenteric ischemia:an NSQIP analysis. Am Surg. 2015;81:1170–1176. doi: 10.1177/000313481508101131. [DOI] [PubMed] [Google Scholar]
- 11.Dhamnaskar SS, Sawarkar PC, Mandal S, Vijaykumaran P. Predictors of mortality in acute mesenteric vascular ischemia with bowel gangrene. Int Surg J. 2016;3:1996–2002. [Google Scholar]


