Figure 6.
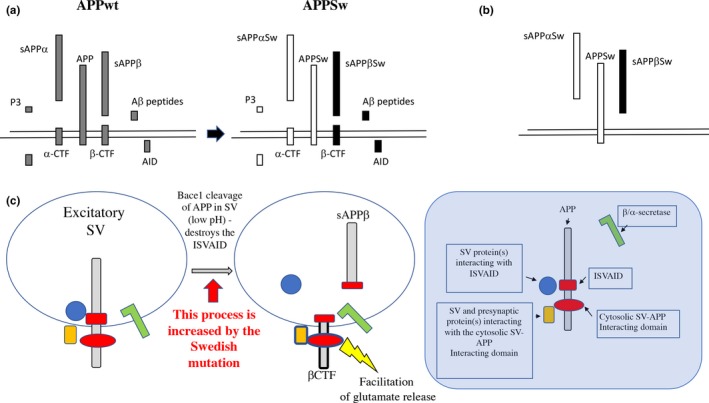
Modeling BAD‐Glu mechanisms and how the Swedish mutation may alter BAD‐Glu. (a) APP undergoes complex proteolysis. In the amyloidogenic proteolytic cascade, APP is cleaved by β‐secretase/BACE1 into sAPPβ and the COOH‐terminal fragment βCTF. Cleavage of βCTF by γ‐secretase produces Aβ peptides and the intracellular domain (AID/AICD). Alternatively, APP is processed by α‐secretase into sAPPα and the COOH‐terminal fragment αCTF. αCTF can be cleaved by γ‐secretase to produce P3 and AID/AICD (Passer et al., 2000; Sisodia & St George‐Hyslop, 2002). The Swedish mutations cause more β‐cleavage with a corresponding increase in direct and indirect metabolites (filled in black), but less α‐cleavage with a corresponding decrease in direct and indirect metabolites (filled in white). In addition, mAPP levels are reduced. The quantitative alterations in one or more of these APP metabolites can participate in BAD‐Glu dysregulation caused by the Swedish mutations. (b) The Swedish mutations cause changes in the primary sequence of several APP metabolites, including APP (APPSw), sAPPβ (sAPPβSw), and sAPPα (sAPPαSw). The qualitative alterations in one or more of these APP metabolites can participate in BAD‐Glu dysregulation caused by the Swedish mutations. (c) APP present in synaptic vesicles can interact with SV proteins and proteins regulating exocytosis via an intraluminal (ISVAID) and a cytosolic (JCasp) domain. Intraluminal and cytosolic interactions may have an opposite effect: the former tunes down glutamate release while the latter facilitates glutamate release. Cleavage of APP by BACE1 inside the ISVAID can abrogate the intravesicular interaction triggering the facilitator function of the cytosolic interaction. Increased β‐cleavage of APPSw can dysregulate BAD‐Glu dysregulation and facilitate glutamate release
