Figure 2.
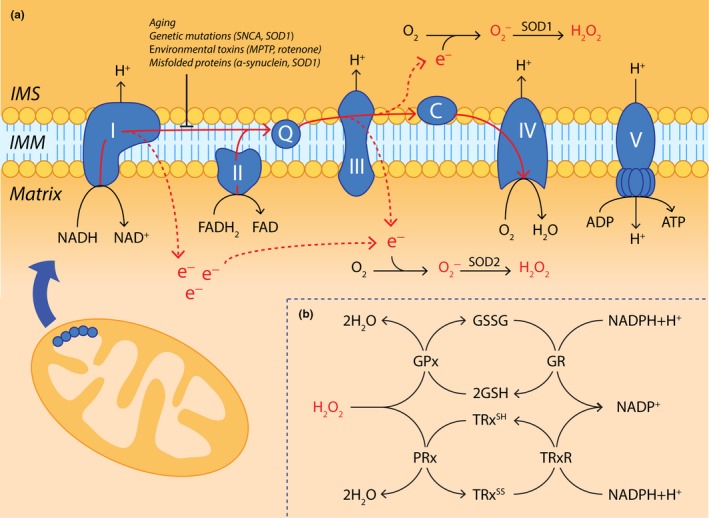
Reactive oxygen species are an inherent by‐product of oxidative phosphorylation in the mitochondrial ETC. (a) Electrons generated by the tricarboxylic acid cycle in the mitochondrial matrix are shuttled to ETC complexes I and II by NADPH and FADH2, respectively. They are then transferred to complex IV of the ETC with the help of inner mitochondrial membrane (IMM) electron shuttles (Q, coenzyme Q; C, cytochrome c) where they reduce molecular oxygen to water, a process which simultaneously drives ATP production by ATP synthase (ETC complex V). A small amount of premature electron leakage occurs naturally during oxidative phosphorylation, whereby electrons bound within ETC complexes I and III diffuse into both the mitochondrial matrix and intermembrane space (IMS). Here, they may cause incomplete reduction of molecular oxygen (O2), generating superoxide radicals (O2 −) that may subsequently be converted to hydrogen peroxide (H2O2) through the action of superoxide dismutase 1 or 2 (SOD1/2). Electron leakage from the electron transport chain is worsened during healthy aging, or by pathogenic factors such as genetic mutations (SNCA, SOD1), environmental toxins (MPTP, rotenone), or misfolded proteins (α‐synuclein, SOD1). (b) Mitochondrial H2O2 levels are regulated by glutathione peroxidase (GPx) and peroxiredoxin (PRx), coupled to the redox cycling of glutathione (GSH/GSSG) and thioredoxin (TRxSH/TRxSS), respectively. While the oxidation component of each cycle is mediated by GPx and PRx, glutathione reductase (GR) and thioredoxin reductase (TRxR) drive NADPH‐dependent glutathione and thioredoxin reduction, respectively, to complete the redox loop
