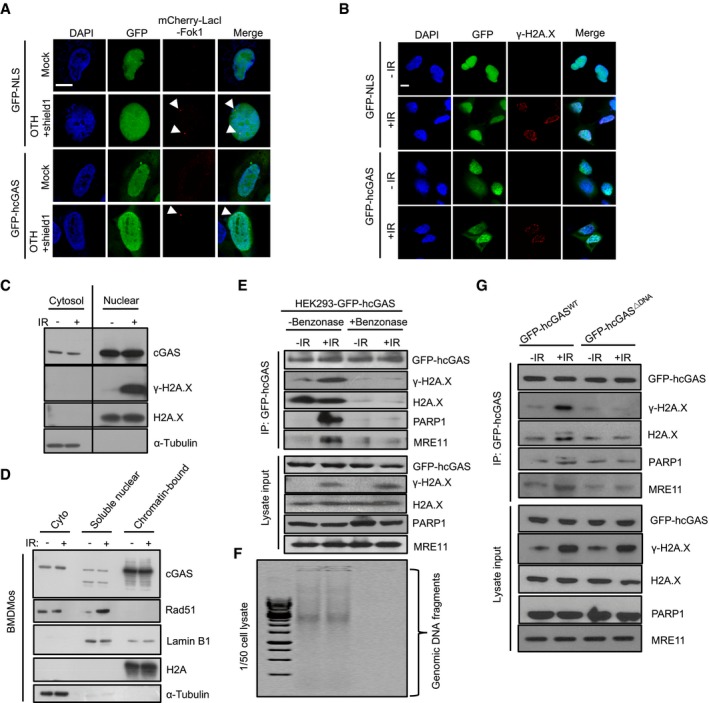
Figure 6. Nuclear cGAS is constantly bound to the chromatin and not specifically recruited to DSB sites.
-
AcGAS is not recruited to DSB sites: Confocal microscopic images of GFP‐NLS‐ or GFP‐hcGAS‐expressing U2OS‐DSB reporter cells incubated (or not) with Shield‐1 and 4‐OHT to induce the expression and translocation of mCherry‐LacI‐FokI (red) to specific DSB sites. Scale bar: 10 μm. The arrowheads indicate DSB sites.
-
BcGAS does not co‐localize with γ‐H2AX at DSB sites: GFP‐NLS‐ or GFP‐hcGAS‐expressing HEK293 cells exposed (or not) to γ‐irradiation (IR: 10 Gy), then stained for γ‐H2AX. Scale bar: 10 μm.
-
C, DNuclear cGAS is mainly chromatin‐bound and remains unaltered upon γ‐irradiation. (C) Cytosolic (cyto) and nuclear fractions of γ‐irradiated (10 Gy, 30 min) BMDMos analyzed for cGAS and indicated molecules. (D) Cytosolic, soluble nuclear, and chromatin fractions from BMDMos were immunoblotted for cGAS and indicated proteins.
-
E–GcGAS co‐isolates with DNA repair proteins because of bound chromatin bridges. (E) Nuclease digestion abrogates the co‐isolation of cGAS and DNA repair proteins: Lysates of control (−IR) and γ‐irradiated (+IR, 10 Gy, 30 min) GFP‐hcGAS‐expressing HEK293 cells were treated (or not) with benzonase before cGAS immunoprecipitation and analysis for indicated proteins. (F) Agarose gel analysis of DNA in corresponding cell lysates in (E). (G) Co‐isolation of cGAS and DNA repair proteins depends on its binding to DNA: cGAS pulldowns along with lysate inputs of control and γ‐irradiated HEK293 cells expressing GFP‐hcGAS or GFP‐hcGASΔDNA probed for indicated proteins.
Source data are available online for this figure.
