A PubMed search using the search term ‘heart rate variability’ in 2019 produced 23,794 results. Heart rate variability (HRV) is popular because it is easily measured and is used as a tool to measure vagal and sympathetic tone to the heart. HRV can even be measured using personal fitness trackers and there are HRV forums on the internet. All of this is despite Zaza and ourselves showing that HRV is primarily affected by heart rate and cannot be used in any simple way to measure cardiac autonomic tone (Rocchetti et al. 2000; Zaza & Lombardi, 2001; Monfredi et al. 2014). There are many ways of measuring HRV. The standard deviation of the normal beat to normal beat interval (SDNN) is popular. HRV can be divided into high and low frequency (HF and LF) components. HF power is used as a measure of cardiac vagal tone and LF power as a measure of cardiac sympathetic tone (Pagani et al. 1997), although others question this (Eckberg, 1997). The problem with HRV is that it is primarily affected by heart rate. There has to be a perturbation (perhaps fluctuations in autonomic tone) that causes HRV. To change heart rate, any perturbation has to produce an ionic current, I per, and this will change (Δ) the rate of change of membrane potential, dV m/dt, during the pacemaker potential:
where C m is the cell capacitance. Consequently, the time taken to reach the threshold potential for triggering an action potential will be altered, i.e. heart rate will be altered. Δ(dV m/dt) (in mV/ms) will be the same regardless of heart rate, but the consequences of Δ(dV m/dt) will be different at different heart rates. Simple geometry will prove to the reader that at a high rate (with steep pacemaker potential) a given Δ(dV m/dt) will produce a relatively small change in the interbeat interval (time required for pacemaker potential to reach threshold potential for triggering an action potential), whereas at a low heart rate (with shallow pacemaker potential) the same Δ(dV m/dt) will produce a larger change in the interbeat interval. An exponential decay‐like relationship between HRV (measured as SDNN) and heart rate predicted by a mathematical model based on this line of reasoning is shown in Fig. 1 A. This predicted relationship fits HRV measured in a wide range of experiments from different species, conditions and laboratories (Fig. 1 A) (Monfredi et al. 2014). To further illustrate the problems with HRV, specific examples will be considered.
Figure 1. Heart rate dependence of HRV.
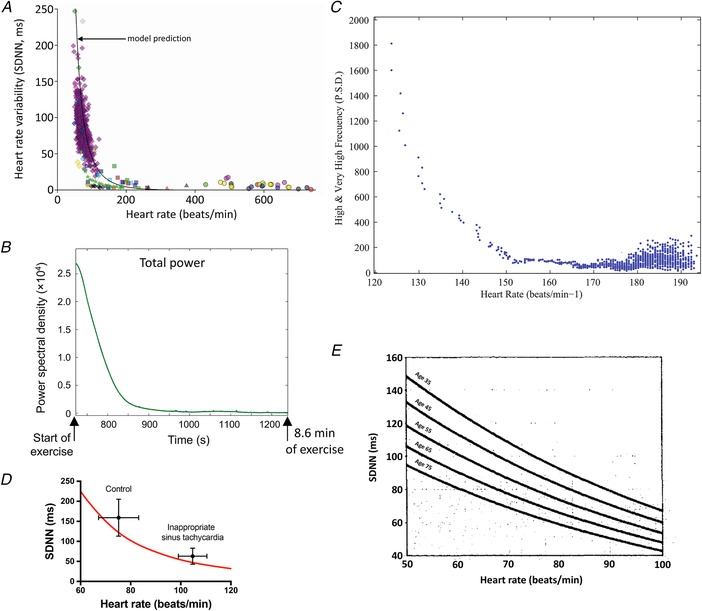
A, relationship between HRV (SDNN) and heart rate from a wide range of studies. It includes data from the healthy conscious human, athletically trained conscious human, conscious human exposed to autonomic blockade, conscious human with heart failure, conscious human with hypertrophic cardiomyopathy, conscious human with myocardial infarction, conscious human heart transplant recipient, conscious mouse (wild type and transgenic), rat, Langendorff‐perfused rabbit and rat hearts, and rabbit sinus node cells (control and exposed to acetylcholine). The black line is the relationship between SDNN and heart rate predicted by the mathematical model of Monfredi et al. (2014). From Monfredi et al. (2014). B, change in HRV (total power) during high intensity exercise (cycloergometer) in an elite cyclist. Data are shown for a single individual, but similar changes were observed in 10 other elite cyclists. From Sarmiento et al. (2013). C, relationship between high frequency power (PSD) and heart rate during high intensity exercise in an elite cyclist. Data are shown for one representative subject. From Sarmiento et al. (2013). D, mean SDNN ± SD plotted as a function of mean heart rate ± SD for a group of 10 patients with inappropriate sinus tachycardia and 10 control age‐ and sex‐matched control subjects. From Castellanos et al. (1998). The red line is the relationship between SDNN and heart rate predicted by the mathematical model of Monfredi et al. (2014). E, fitted regression lines of 2 h SDNN as a function of age for 2722 male and female human subjects. Data are shown for five specific ages (35, 45, 55, 65 and 75 years) at mean heart rates between 50 and 100 beats/min. From Tsuji et al. (1996). PSD, power spectral density.
HRV during exercise
During exercise, heart rate increases as a result of a decrease in vagal tone and increase in sympathetic tone (Michael et al. 2017). On this basis, a decrease in HF power and increase in LF power is expected, whereas a dramatic decrease in all HRV measures is observed (Casadei et al. 1995; Michael et al. 2017). Figure 1 B shows an example of total power from Sarmiento et al. (2013), who conclude, based on similar falls in HF and LF power that during exercise, ‘parasympathetic control may have disappeared, or fallen to minimal levels, whilst sympathetic activity may also have been seriously compromised’. The possibility that sympathetic tone is seriously compromised during exercise is untenable and this illustrates the lack of correspondence between HRV and cardiac autonomic tone. We are not the first to recognise this (Casadei et al. 1995). Instead, all indices of HRV are expected to fall during exercise simply because heart rate increases during exercise; this explains why all indices fall in qualitatively the same way. In Fig. 1 C, HF power in the study of Sarmiento et al. (2013) is plotted against heart rate and there is an exponential decay‐like relationship similar to that predicted by the mathematical model described above (Fig. 1 A); there is also an inverse relationship between both LF and total power and heart rate. It is concluded that the decay in HRV indices during exercise is the result of the concurrent increase in heart rate and not a consequence of changes in autonomic tone.
HRV in inappropriate sinus tachycardia
Patients with inappropriate sinus tachycardia (IST) have an abnormally high resting heart rate with symptoms ranging from slight to severe (Castellanos et al. 1998). Castellanos et al. (1998) reported that patients with IST have reduced HRV (Fig. 1 D), which they suggested was the result of a decrease in vagal tone; this then could explain the elevated heart rate. However, Fig. 1 D shows that the decrease in HRV in IST is well explained by the increase in heart rate in these patients (red line in Fig. 1 D is the predicted relationship between HRV and heart rate from model described above). Therefore, there is no reason to believe that the high heart rate in IST is the result of a decrease in vagal tone. Consistent with this, patients with IST are treated with ivabradine to reduce the heart rate to normal (Achike & DeAntonio, 2018); this suggests that the cause lies with ion channels and HCN4 in particular. HCN4 is blocked by ivabradine and is the major isoform responsible for the funny current (I f), arguably the most important pacemaker current in the heart (DiFrancesco, 2010).
HRV in athletes and circadian rhythm in heart rate
If HRV cannot be used in any simple way to measure cardiac autonomic tone, a fresh look has to be taken at heart rate changes attributed, largely based on HRV, to changes in cardiac autonomic tone. We have reinvestigated the resting bradycardia in athletes, which is widely attributed to high vagal tone based on an increase in HRV in athletes. In the human and mouse, exercise training results in bradycardia and we have shown that this is not the result of high vagal tone (for example, bradycardia is still present after autonomic blockade) and instead is due to downregulation of HCN4 and I f (D'Souza et al. 2014, 2017). We argue that it is the HCN4‐dependent bradycardia in athletes that is responsible for the increase in HRV. Consistent with this, loss‐of‐function mutations in HCN4 lead to bradycardia and increased HRV (Hategan et al. 2017). Moreover, the exercise training‐induced bradycardia in mice is reversed by restoring HCN4 (D'Souza et al. 2017). We are also reinvestigating the circadian rhythm in resting heart rate attributed to a circadian rhythm in cardiac autonomic tone based on HRV studies and we have obtained evidence that a circadian rhythm in HCN4 and I f is at least involved (Wang et al. 2016).
HRV in ageing and summary
In summary, HRV is primarily determined by heart rate and cannot be used in any simple manner to determine cardiac autonomic tone. It is not even known whether autonomic nerve activity affects HRV – previously, this will have been tested by investigating the effect of autonomic blockade on HRV, but of course autonomic blockade will affect the heart rate and therefore HRV. Is there any way to separate the effect of heart rate on HRV from the effect of an independent factor on HRV? We know of one study in which this is possible: as part of the Framingham Heart study, Tsuji et al. (1996) showed, based on data from 2722 human subjects, that heart rate and ageing were independent determinants of HRV (Fig. 1 E). Therefore, it is possible, but it requires large cohorts of subjects. In Fig. 1 A, the experimental data are scattered around the predicted relationship between HRV and heart rate – the scatter could be the result of heart rate‐independent factors, but a lot of work would be required to test this.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘LastWord’. Please email your comment, including a title and a declaration of interest, to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Additional information
Competing interests
None declared.
Author contributions
All authors contributed to the conception and design of the work, acquisition and interpretation of data for the work and drafting the work and revising it critically for important intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the British Heart Foundation (BHF; to M. R. Boyett, RG/18/2/33392).
Supporting information
Comments
Last words by Malik et al.
Last words by Boyett & D'Souza.
Biographies
Mark Boyett received a PhD in Physiology from University College London in 1977, was appointed a Lecturer in Physiology at the University of Leeds in 1978 and was appointed the Professor of Cardiac Electrophysiology at the University of Manchester in 2005.

Yanwen (Wendy) Wang is a Research Fellow at the University of Manchester. After receiving a PhD in 2013 from the University of Manchester, she was a Research Associate at the University of Oxford before returning to Manchester in 2015.
Alicia D'Souza is a British Heart Foundation Intermediate Basic Science Research Fellow at the University of Manchester. After receiving a PhD in 2011 from the University of Central Lancashire, she moved to Manchester. Her work has attracted numerous international prizes and she was The Physiological Society's inaugural R Jean Banister Prize lecturer.
Edited by: Francisco Sepúlveda & Michael Shattock
The authors work as a group studying heart rate control by the pacemaker of the heart, the sinus node.
Linked articles: This article is part of a CrossTalk debate. Click the links to read the other articles in this debate: https://doi.org/10.1113/JP277963, https://doi.org/10.1113/JP277500 and https://doi.org/10.1113/JP277962.
References
- Achike O & DeAntonio H (2018). Ivabradine and inappropriate sinus tachycardia: a funny target for an inappropriate disease. J Am Coll Cardiol 71 (suppl.), 2606. [Google Scholar]
- Casadei B, Cochrane S, Johnston J, Conway J & Sleight P (1995). Pitfalls in the interpretation of spectral analysis of the heart rate variability during exercise in humans. Acta Physiol Scand 153, 125–131. [DOI] [PubMed] [Google Scholar]
- Castellanos A, Moleiro F, Chakko S, Acosta H, Huikuri H, Mitrani RD & Myerburg RJ (1998). Heart rate variability in inappropriate sinus tachycardia. Am J Cardiol 82, 531–534. [DOI] [PubMed] [Google Scholar]
- D'Souza A, Bucchi A, Johnsen AB, Logantha SJRJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dobryznski H, DiFrancesco D, Morris GM & Boyett MR (2014). Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun 5, 3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza A, Pearman CM, Wang Y, Nakao S, Logantha SJRJ, Cox C, Bennett H, Zhang Y, Johnsen AB, Linscheid N, Poulsen PC, Elliott J, Coulson J, McPhee J, Robertson A, Da Costa Martins PA, Kitmitto A, Wisløff U, Cartwright EJ, Monfredi O, Lundby A, Dobrzynski H, Oceandy D, Morris GM & Boyett MR (2017). Targeting miR‐423‐5p reverses exercise training‐induced HCN4 channel remodeling and sinus bradycardia. Circ Res 121, 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D (2010). The role of the funny current in pacemaker activity. Circ Res 106, 434–446. [DOI] [PubMed] [Google Scholar]
- Eckberg DL (1997). Sympathovagal balance: a critical appraisal. Circulation 96, 3224–3232. [DOI] [PubMed] [Google Scholar]
- Hategan L, Csányi B, Ördög B, Kákonyi K, Tringer A, Kiss O, Orosz A, Sághy L, Nagy I, Hegedűs Z, Rudas L, Széll M, Varró A, Forster T & Sepp R (2017). A novel ‘splice site’ HCN4 gene mutation, c.1737+1 G>T, causes familial bradycardia, reduced heart rate response, impaired chronotropic competence and increased short‐term heart rate variability. Int J Cardiol 241, 364–372. [DOI] [PubMed] [Google Scholar]
- Michael S, Graham KS & Davis GMO (2017). Cardiac autonomic responses during exercise and post‐exercise recovery using heart rate variability and systolic time intervals – a review. Front Physiol 8, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H & Boyett MR (2014). Biophysical characterisation of the under‐appreciated and important relationship between heart rate variability and heart rate. Hypertension 64, 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C & Somers VK (1997). Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95, 1441–1448. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Malfatto G, Lombardi F & Zaza A (2000). Role of the input/output relation of sinoatrial myocytes in cholinergic modulation of heart rate variability. J Cardiovasc Electrophysiol 11, 522–530. [DOI] [PubMed] [Google Scholar]
- Sarmiento S, García‐Manso JM, Martín‐González JM, Vaamonde D, Calderón J & Da Silva‐Grigoletto ME (2013). Heart rate variability during high‐intensity exercise. J Syst Sci Complex 26, 104–116. [Google Scholar]
- Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL & Levy D (1996). Determinants of heart rate variability. J Am Coll Cardiol 28, 1539–1546. [DOI] [PubMed] [Google Scholar]
- Wang Y, D'Souza A, Johnsen A, Olieslagers S, Cox C, Bucchi A, Wegner S, Gill E, Cartwright E, Wisloff U, Martins PDC, Difrancesco D, Dobrzynski H, Piggins H & Boyett M (2016). Circadian rhythm in heart rate is due to an intrinsic circadian clock in the sinus node. Eur Heart J 37 (Abstract suppl.), 618. [Google Scholar]
- Zaza A & Lombardi F (2001). Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovasc Res 50, 434–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comments
Last words by Malik et al.
Last words by Boyett & D'Souza.


