Table 1.
Establishment of optimal conditions.
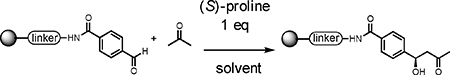 | |||
|---|---|---|---|
| Entry | Solvent | Temp (°C) | Conversion (%) |
| 1 | acetonitrile | 4 | 0 |
| 2 | acetonitrile | 20 | 2 |
| 3 | NMP | 4 | 11 |
| 4 | NMP | 20 | 42 |
| 5 | DMSO | 4 | 75 |
| 6 | DMSO | 20 | 95 |
| 7 | DMF | 4 | 4 |
| 8 | DMF | 20 | 35 |
Reaction conditions: Reactions were carried out using 1 eq. of (S)-proline (relative to the immobilized aldehyde (0.006 mmol)) and acetone (100 eq, 44 μL) in 120 μL DMSO. Conversion was determined from cleaved crude mixture by monitoring LC-MS analysis of the crude product after reductive cleavage from the beads., This was calculated by measuring the ratio of the remaining aldehyde peak and that of an internal standard (the equivalent molecule lacking a formyl group) (see supporting info).
