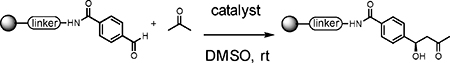
Table 2.
Comparison of various proline-derived catalysts.
 | ||||
|---|---|---|---|---|
| Entry | catalyst | amount of catalyst (eq) | Conversion (%) | ee (%) |
| 1 | 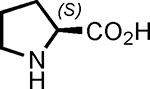 |
1 | 99 | 73 |
| 2a |  |
1 | 99 | 70 |
| 3 | 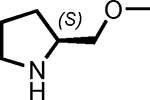 |
5 | 98 | 8 |
| 4 | 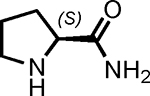 |
3 | 99 | 1 |
Reaction conditions: Reactions were carried out using organocatalyst and acetone (100 eq, 44 μL) to resin loading (0.006 mmol) in 120 μL of DMSO for 16 hours. Conversion was determined from cleaved crude mixture by monitoring LC-MS, calculated by two peak ratio between starting aldehyde and inactive benzoic ring which is used for internal standard. (see supporting info)
Reaction was completed in 4h.
