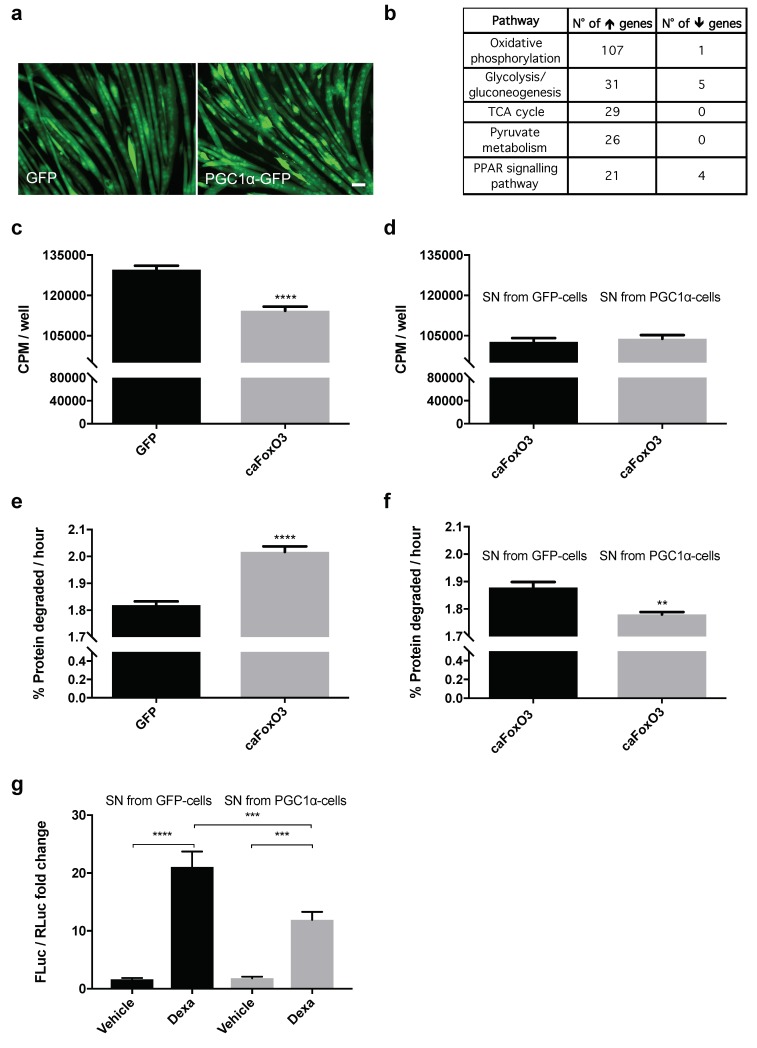
Figure 1.
Peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α)-overexpressing myotubes secrete some anti-catabolic factors. Representative fields of four-day differentiated myotubes infected for 48 h with Green Fluorescent Protein (GFP) or PGC1α-GFP-expressing myotubes are shown. Scale bar, 25 μm (a). Enriched pathways from Kyoto Encyclopedia of Genes and Genomes (KEGG) (p < 0.05) are shown from the microarray differential expression analysis, which involves the mitochondrial function, with the number of upregulated and downregulated genes belonging to each pathway (b). Rates of overall protein synthesis and long-lived protein degradation were measured in myotubes transfected on the fourth day of differentiation with GFP or constitutively active Forkhead box-containing subfamily O3 (caFoxO3)-expressing viruses. Infection of myotubes for 48 h with adenoviruses encoding for caFoxO3 reduces protein synthesis (c) and increases protein degradation of long-lived proteins (e). Unpaired t-test, **** p ≤ 0.0001, n = 6. GFP-expressing adenoviruses are used as control. Supernatants (SN) from myotubes infected for 72 h with PGC1α-encoding adenoviruses do not alter protein synthesis (d) but restrain caFoxO3-induced protein degradation (f). Unpaired t-test, **, p ≤ 0.01, n = 6. CPM, counts per minute. The dexamethasone-induced MuRF1 promoter-Firefly Luciferase (FLuc) signal is reduced in myoblasts exposed to SN from PGC1α-expressing cells but not from GFP-expressing ones. Myoblasts were transfected with MuRF1 promoter-FLuc plasmid and HSV-thymidine kinase (TK) promoter-Renilla Luc plasmid (RLuc) and 24 h later treated for 24 h with 10 μM dexamethasone and the aforementioned SN. The results of three independent experiments are shown (g). One-way analysis of variance (ANOVA) followed by Tukey’s test, *** p ≤ 0.001, **** p ≤ 0.0001, n = 6.