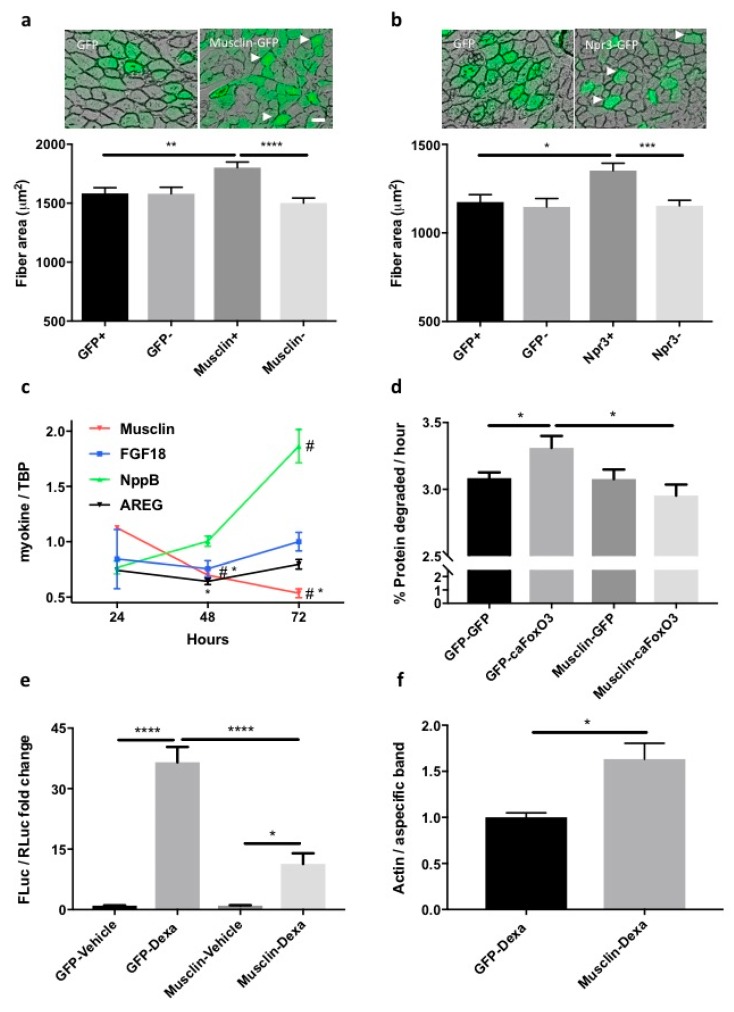
Figure 5.
Overexpression of musclin (or its receptor) preserves the fiber area of Tibialis Anterior (TA) during C26 tumor growth in mice, reduces the proteolysis of long-lived proteins in atrophying myotubes, and restrains MuRF1-based signaling in dexamethasone-treated cells. A representative transverse section of fibers electroporated with musclin-GFP-carrying plasmids (a) or Npr3-GFP-expressing ones (b) from C26-bearing mice is shown. Scale bar, 50 μm. Arrowheads indicate enlarged fibers as example. The mean cross-sectional area (CSA) of fibers electroporated with the indicated plasmids is plotted for C26-bearing mice, indicating preserved fiber area in musclin or Npr3-expressing fibers (a,b). We analyzed 5–6 legs for a total of 102 GFP-expressing fibers and 102 GFP-negative ones, 140 musclin-positive and 140 musclin-negative fibers, 171 Npr3-positive and 171 Npr3-negative fibers. One-way ANOVA followed by Tukey’s test, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Myotubes infected for 24, 48 and 72 h with adenoviruses for GFP of caFoxO3 and the expression of the indicated myokines is shown as the fold change over control. Only musclin expression is reduced by caFoxO3 overexpression. TBP was used as housekeeping gene (c). One-way ANOVA with repeated measures followed by Newman–Keuls post hoc test. * p ≤ 0.05 vs. controls (GFP-expressing myotubes); # p ≤ 0.05 vs. 24 h (musclin) or vs. both other timepoints (NppB), n = 3. Three-day differentiated myotubes were transfected with plasmids for GFP or musclin and the next day, these cells were further infected with adenoviruses for GFP or caFoxO3, as indicated. The caFoxO3-induced rates of degradation of long-lived proteins are lowered by co-expressed musclin (d). One-way ANOVA followed by Tukey’s test, * p ≤ 0.05, n = 5–6. The dexamethasone-induced MuRF1 promoter-FLuc signal is reduced in myoblasts expressing musclin or Npr3 but not in GFP-expressing ones. Myoblasts treated for 24 h with 10 μM dexamethasone had first been transfected with MuRF1 promoter-FLuc plasmid and TK promoter-RLuc plasmid in combination with the other plasmids. The results of one representative experiment repeated three times are shown (e). One-way ANOVA followed by Dunnett’s test, * p ≤ 0.05, **** p ≤ 0.0001, n = 3. Densitometric analysis shows that actin protein is spared in dexamethasone-treated myotubes expressing musclin with respect to GFP-expressing ones (f). A non-specific band of about 300 kDa found with actin antibody was used as loading control. Unpaired t test, * p ≤ 0.05, n = 3.