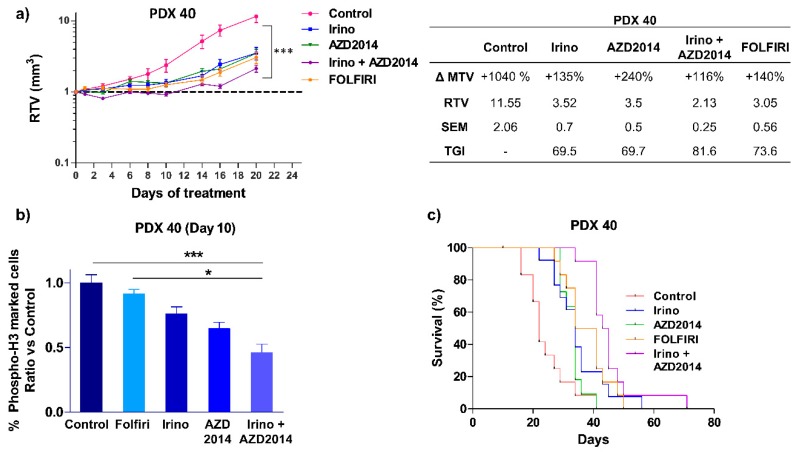
Figure 5.
In vivo effect of irinotecan (Irino) and AZD2014 combination on PDX 40 tumor growth. (a) Relative tumor volume (mean RTV ± standard deviation) as a function of time for the PDX 40. Each group consisted of 7 subcutaneous xenografted mice. The table summarizes the ΔMTV, RTV and TGI for each group between the beginning and the end of treatment. ΔMTV (%): variation of the mean tumor volume between day 1 and the end of the treatments. RTV: Relative tumor volume. SEM: Standard error of mean. TGI (%): Tumor growth inhibition. (b) Quantification of cells positive for phospho-histone H3 in PDX 40, 10 days after initiation of treatment. Data are expressed as a relative ratio versus the control group. (c) Kaplan-Meier analysis of mouse survival for PDX 40 in each group of treatment. An event corresponds to the ethical sacrifice of the mouse due to a tumor volume reaching 1500 mm3 or because of tumor ulceration. The treatment schedules are as follows: irinotecan (Irino) (IP, 10 mg/kg/day q5d); AZD2014 (Oral gavage, 20 mg/kg BID, 2 days on/5 days off); FOLFIRI (5FU: 50 mg/kg, q7d, IP, LV: 90 mg/kg, q7d, IP, irinotecan 10 mg/kg, q5d, IP). Control mice received no treatment. * p < 0.05, *** p < 0.0005.