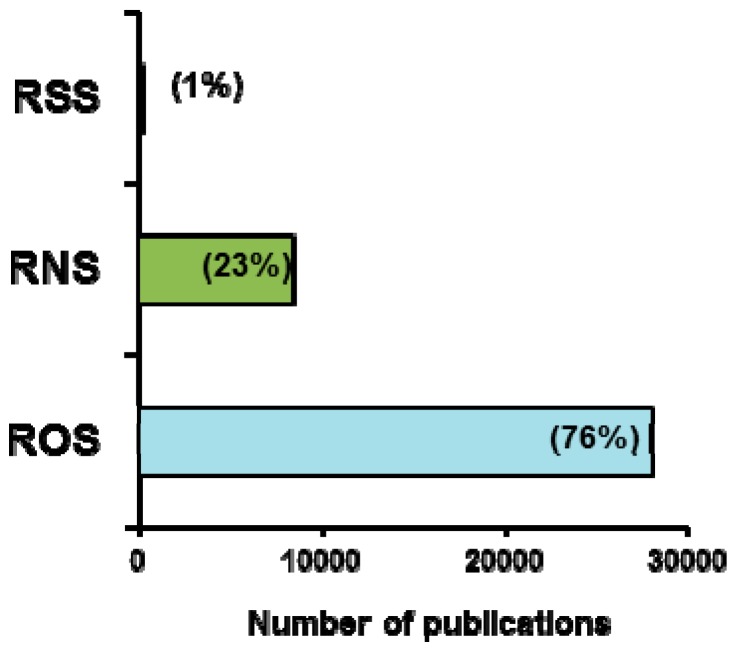
Nitric oxide (NO) and hydrogen sulfide (H2S) are two gasotransmitters endogenously generated in plant cells. Each belongs to a family of related molecules called reactive nitrogen and sulfur species (RNS and RSS), respectively, which perform multiple functions in the physiology of plants. NO and H2S appear to share various plant tasks and are involved in a wide range of physiological processes, including seed germination, root architecture, plant growth and development, stomatal movement, senescence and fruit ripening, as well as in the mechanism of response to environmental stresses [1,2,3,4]. Their mechanism of action is mainly through protein posttranslational modifications, such as S-nitrosation, nitration and persulfidation, which affect the redox status and function of target proteins [5,6,7]. NO and H2S, which mediate several signaling networks, are key elements in the biochemistry and physiology of plants. Furthermore, increasing experimental data demonstrate crosstalk between these molecules and reactive oxygen species (ROS) metabolism. However, information on the plant cell metabolism of NO and H2S is still limited compared to that on ROS metabolism. A simple search in the PubMed database using the terms plant and the corresponding reactive species enables one to access all of the relevant information published in this field, wherein RNS and RSS account for only 23% and 1% of the total, as compared to 76% for ROS (Figure 1).
Figure 1.
Number of publications related with reactive oxygen, nitrogen and sulfur species (ROS, RNS and RSS, respectively) and plants found in PubMed database.
Advances in scientific knowledge are made through the accumulation of information published in research papers. This special issue on “Nitric oxide (NO) and hydrogen sulfide (H2S) in higher plants under physiological and stress conditions” aims to provide up-to-date research in the area of NO, H2S and ROS metabolism. One review and six research papers have been brought together in this issue which offers new insights into the role played by these signaling molecules. The review by Kolbert et al. [8] provides a broad perspective on the interaction between NO and the phytohormone ethylene, which is either synergistic or antagonistic depending on the physiological process ranging from seed germination and development to senescence and fruit ripening, as well as the mechanism of response to stressful conditions. The authors point out that, while the NO signal cascade mainly takes place through protein posttranslational modifications, the effects of ethylene are initiated by a specific receptor. On the other hand, the six research papers study different aspects of the involvement of NO and H2S in beneficial interactions between microorganisms and plant roots, as well as in the ripening process of climacteric and non-climacteric fruits. Using genetic techniques, Fukusome et al. [9] analyze how the level of NO level regulated by heme-containing protein phytoglobin 1 (Glb1) beneficially affects nodule formation during invasions of Lotus japoniscus roots by Mesorhizobium loti, especially under hypoxic stress conditions triggered by flooding, and also demonstrate a concomitant reduction in ROS production. Azolla pinnata is a water fern characterized by a rapid root abscission phenomenon in response to certain environmental stimuli. The study by Yamasaki et al. [10] uses this model to analyze the RSS metabolism with the aid of l- and d-cysteine as the potential source of H2S, as well as a battery of new H2S donors including polysulfides. Their findings demonstrate that d-cysteine is a major substrate for H2S production in Azolla pinnata which is mediated by d-cysteine desulfhydrase activity, while the root abscission phenomenon is a good model for evaluating the efficiency of different H2S donors in aqueous solutions. The study by Chu-Puga et al. [11] shows that, during the ripening of non-climacteric sweet pepper (Capsicum annuum L) fruit, there is an increase in the content of lipid peroxidation, a marker of oxidative stress, which is associated with an increase in superoxide-generating respiratory burst oxidase homolog (Rboh) activity. Using in vitro assays, they also show that Rboh activity is inhibited by NO, peroxynitrite (ONOO−) and reduced glutathione (GSH), suggesting that this activity is modulated by posttranslational modifications, such as S-nitrosation, tyrosine nitration and glutathionylation, and that fruit ripening is associated with nitro-oxidative stress. Rodriguez-Ruiz et al. [12], who carried out a biochemical characterization of the peroxisomal antioxidant catalase in pepper fruits, found that this catalase has an atypical molecular mass, suggesting that, unlike the typical tetrameric catalase present in higher plants, it is a homodimer. By analyzing in vitro pepper catalase, the authors found that the activity of this antioxidant enzyme is negatively modulated by S-nitrosation and nitration. Lokesh et al. [13] study how the exogenous application of NO triggers polyamine accumulation in postharvest banana (Musa acuminate) fruit, apparently via the arginine-mediated route. Meanwhile, the study by Geng et al. [14] shows that H2S content is modulated by NO in peach (Prunus persica) fruit during cold storage, whose quality is adversely affected by the chilling injury phenomenon.
In summary, this special issue provides novel information on the complex relationship between H2S and NO which also affect the metabolism of ROS. It is worth noting that some research included in this issue focuses on plant species of agronomic interest, such as climacteric and no-climacteric fruits, whose ripening and quality can be affected during postharvest storage by the exogenous application of NO and/or H2S.
Funding
F.J.C. research work is supported by the ERDF-cofinanced grant from the Ministry of Economy and Competitiveness (AGL2015-65104-P) and Junta de Andalucía (group BIO192), Spain.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Yamasaki H., Cohen M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide. 2016;55:91–100. doi: 10.1016/j.niox.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Fancy N.N., Bahlmann A.K., Loake G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017;40:462–472. doi: 10.1111/pce.12707. [DOI] [PubMed] [Google Scholar]
- 3.Corpas F.J., González-Gordo S., Cañas A., Palma J.M. Nitric oxide and hydrogen sulfide in plants: which comes first? J. Exp. Bot. 2019;70:4391–4404. doi: 10.1093/jxb/erz031. [DOI] [PubMed] [Google Scholar]
- 4.Corpas F.J. Hydrogen sulfide: A new warrior against abiotic stress. Trends Plant Sci. 2019 doi: 10.1016/j.tplants.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Begara-Morales J.C., Sánchez-Calvo B., Chaki M., Valderrama R., Mata-Pérez C., Padilla M.N., Corpas F.J., Barroso J.B. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs) Front Plant Sci. 2016;7:152. doi: 10.3389/fpls.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asgher M., Per T.S., Masood A., Fatma M., Freschi L., Corpas F.J., Khan N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. Int. 2017;24:2273–2285. doi: 10.1007/s11356-016-7947-8. [DOI] [PubMed] [Google Scholar]
- 7.Aroca A., Gotor C., Romero L.C. Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci. 2018;9:1369. doi: 10.3389/fpls.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolbert Z., Feigl G., Freschi L., Poór P. Gasotransmitters in Action: Nitric oxide-ethylene crosstalk during plant growth and abiotic stress responses. Antioxidants (Basel) 2019;8:167. doi: 10.3390/antiox8060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukudome M., Watanabe E., Osuki K.I., Uchi N., Uchiumi T. Ectopic or over-expression of class 1 phytoglobin genes confers flooding tolerance to the root nodules of Lotus japonicus by scavenging nitric Oxide. Antioxidants (Basel) 2019;8:206. doi: 10.3390/antiox8070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki H., Ogura M.P., Kingjoe K.A., Cohen M.F. D-Cysteine-induced rapid root abscission in the water fern Azolla pinnata: Implications for the linkage between D-amino acid and Reactive Sulfur Species (RSS) in plant environmental responses. Antioxidants (Basel) 2019;8:411. doi: 10.3390/antiox8090411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu-Puga Á., González-Gordo S., Rodríguez-Ruiz M., Palma J.M., Corpas F.J. NADPH oxidase (Rboh) activity is up regulated during sweet pepper (Capsicum annuum L.) fruit ripening. Antioxidants (Basel) 2019;8:9. doi: 10.3390/antiox8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Ruiz M., González-Gordo S., Cañas A., Campos M.J., Paradela A., Corpas F.J., Palma J.M. Sweet Pepper (Capsicum annuum L.) fruits contain an atypical peroxisomal catalase that is modulated by Reactive Oxygen and Nitrogen Species. Antioxidants (Basel) 2019;8:374. doi: 10.3390/antiox8090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lokesh V., Manjunatha G., Hegde N.S., Bulle M., Puthusseri B., Gupta K.J., Neelwarne B. Polyamine induction in postharvest banana fruits in response to NO donor SNP occurs via L-arginine mediated pathway and not via competitive diversion of S-adenosyl-l-methionine. Antioxidants (Basel) 2019;8:358. doi: 10.3390/antiox8090358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng B., Huang D., Zhu S. Regulation of Hydrogen sulfide metabolism by nitric oxide inhibitors and the quality of peaches during cold storage. Antioxidants (Basel) 2019;8:401. doi: 10.3390/antiox8090401. [DOI] [PMC free article] [PubMed] [Google Scholar]