Figure 2.
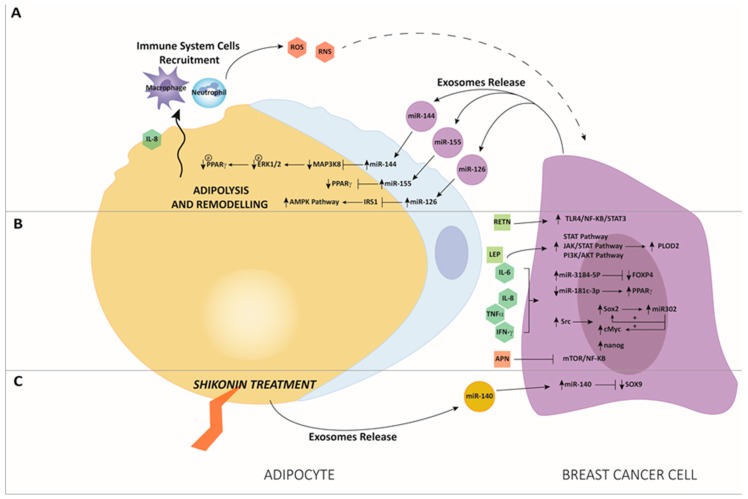
(A) After co-culture between adipocytes and breast cancer (BC) cells, high levels of miR-144 and miR-126 were released by exosomes by BC cells. This miRs delivery leads to final adipolysis and remodeling of adipose tissue driven by miR-144 through the downregulation of Mitogen-Activated Protein Kinase Kinase Kinase 8/Mitogen-Activated Protein Kinase 3/1/Peroxisome Proliferator Activated Receptor Gamma (MAPK3/ERK1/2/ pPPARγ) and by miR-126 through the Protein Kinase AMP-Activated Catalytic Subunit Alpha 1 (AMPK)/ autophagy pathway, causing adipocyte-induced tumor growth [77]. BC-derived exosomes after adipocytes interaction carry a higher level of miR-155, which promotes adipocytes differentiation and remodeling in surrounding adipocytes targeting pPPARγ, thus facilitating tumor progression [76]. Increased adipocyte-derived interleukin-8 (IL-8) is responsible for neutrophils and macrophages recruitment, leading to the production of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), which act as a threat to tumor initiation. (B) Interaction of adipocytes with BC cells increase the release of proinflammatory cytokines and chemokines, leading to an upregulation of miR-3184-5p and downregulation of miR-181c-3p in BC cells, with the modulation of their respective target genes, forkhead box P4 (FOXP4) and PPARα; increased release of adipocyte-derived proinflammatory cytokines and adipokins such as leptin (LEP), IL-6, IL-8 and tumor necrosis factor alpha (TNFα) is responsible for the induction of janus kinase/signal transducers and activators of transcription (JAK/STAT) and through phosphoinositide 3-kinase/protein kinase B (PIK3/AKT) pathways. Resistin (RETN) promotes BC progression through the Toll like receptor 4/nuclear factor kappa-light-chain-enhancer of activated B cells/ signal transducer and activator of transcription 3 (TLR4/ NF-κB /STAT3) axis. As a result, EMT, migration and invasion pathways are enhanced in BC cells [72]. Likewise, after adipocytes and BC cells co-culture, there is an increase of proinflammatory cytokines secretion, with an activation of Src that upregulates SRY-Box transcription factor 2 (SOX2), cMYC and Nanog. SOX2 induces miR-302b transcription that, in turn, further stimulates cMYC and SOX2 expression, increasing the cytokine-induced cancer stem cell-like properties [73]. Conversely, adiponectin (APN) has anti-tumoral properties by inhibiting mammalian target of rapamycin (mTOR) and NF-κB pathways. (C) Treatment with the anti-tumor compound shikonin (SK). SK-treated adipocytes release high levels of miR-140, which block tumor progression of co-cultured BC cells targeting SOX9 signaling [74].